Maintaining an Inspection Ready eTMF: Active TMF Management at the Veeva R&D Summit
Veeva recently hosted its annual R&D Summit, which brought together more than 100 trial master file professionals from across the industry – from large pharma to small biotechs to CROs – to share challenges and strategies related to TMF management. When talking among colleagues or listening to questions, it was quite clear that maintaining inspection readiness was top of mind for most attendees this year. It was also clear that a new approach to TMF management – active TMF management – is being embraced by the industry to improve inspection readiness and meet regulatory expectations.
Historically, organizations have leveraged a ‘passive’ TMF operating model where documents are uploaded into the system only after they are finalized. The underlying processes are based on age-old paper processes even if they have updated to an electronic format. Work is still conducted outside the system, documents are stored in multiple locations, content is not tracked in progress, and no single system serves as the eTMF.
Whereas an active approach to TMF management ensures all TMF documents, related information, and processes are managed in the same system, at the same time, as they are being executed. TMF composition is an automatic result of an executed clinical process and the TMF is maintained in a constant state of inspection readiness. As a result, compliance is continuous, not an end state.
Data presented at the conference from Veeva’s TMF Maturity Model, which measures how active an organization’s trial master file strategy is, shows an industry rapidly reaching toward active TMF management.
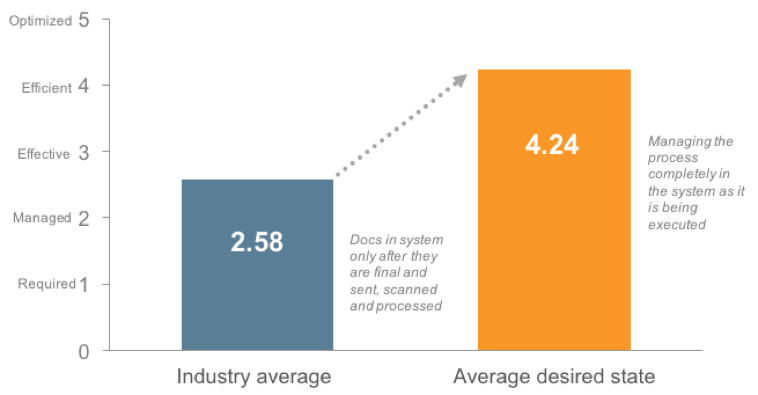
Figure 1: Current industry TMF maturity average and desired industry TMF maturity average. Data from Veeva TMF Maturity Model.
Much of this active TMF movement seems to be driven by guidance from the MHRA and their 2014 updated definition of a critical GCP finding to include incomplete TMFs. A passive TMF model requires a massive effort to prepare for an inspection, and even then the TMF is often inadequate for regulatory review (at the MHRA GCP Symposium in September, a senior GCP inspector reported that 31% of TMF inspections last year required extra days because of incomplete or inaccessible TMFs). While the active model is still relatively new, attendees at the Summit that have already adopted and experienced an MHRA inspection reported substantial improvements.