Streamline Variation Management by Connecting Quality and Regulatory
Each year, a large biopharma company can evaluate more than 40,000 change requests and approve approximately 15,000. Most of these changes have significant quality and regulatory implications, which both teams need to understand and monitor as part of the variation management process.
Unfortunately, information gaps often limit collaboration. Separate quality and regulatory systems restrict visibility into regulatory impacts and the status of regulatory filings. As a result, companies may lack complete, up-to-date information when authorizing a change or making a downstream product shipping decision. This, in turn, can lead to added costs, longer cycle times, and increased risk of non-compliance.
Unified platform bridges the information gap
By shifting to a unified platform, biopharma companies can automate the exchange of information between quality and regulatory systems and streamline the overall variation management process. There are two critical touchpoints where companies can improve collaboration: regulatory impact assessments and regulatory approval tracking.
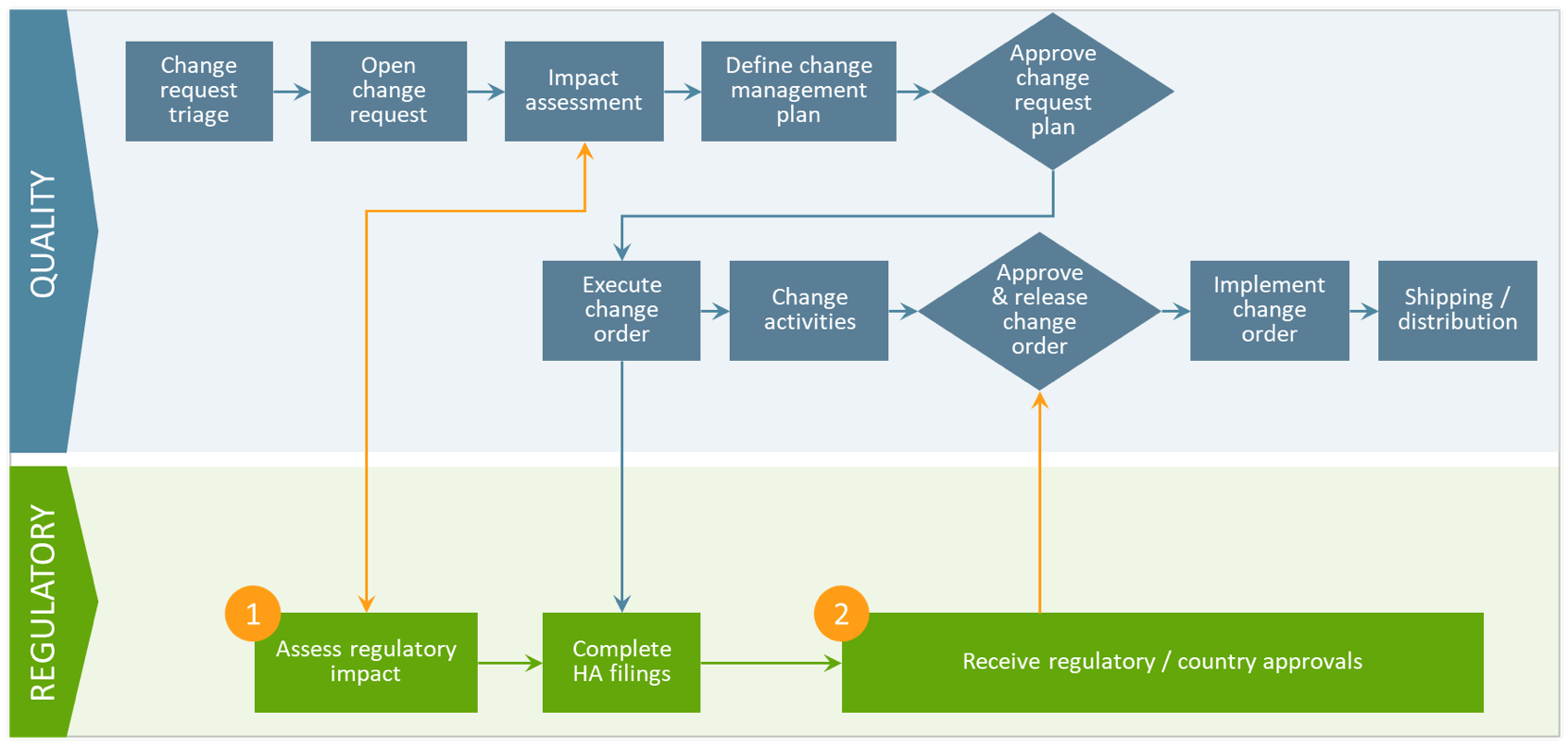
- Regulatory impact assessment
Regulatory teams rely on local dispositioning of proposed changes to accurately assess global implications. With a unified platform, regulatory teams can quickly identify impacted product licenses and seamlessly transmit that data to quality so they can make an informed go/no-go decision. - Regulatory approval tracking
When a change request plan is approved, it triggers the next step in the variation management process where regulatory prepares, submits, and tracks required health authority filings. With real-time visibility into submission status and health authority approvals, quality can gain a clearer picture of when and where the final change should be executed.
Connecting quality and regulatory information through a unified platform enables teams to harmonize an otherwise disjointed variation management process and drives several benefits including:
- Greater compliance: Real-time visibility into regulatory approvals helps companies maintain compliance with health authorities around the world.
- Shorter cycle time: Connecting and automating the variation management process avoids delays due to email exchanges, spreadsheet searches, and redundant data entry.
- Informed shipping decisions: Trusted regulatory data helps improve the timing of product releases and ensure continued patient access to critical drugs.
- Cost optimization: Regulatory status information drives decisions about production changeovers and inventory levels across impacted markets.
To learn more about how companies can bridge the information gap between quality and regulatory systems, watch the on-demand webinar “Streamline Change Control and Variation Management.”