Three Best Practices for a Successful RIM Implementation
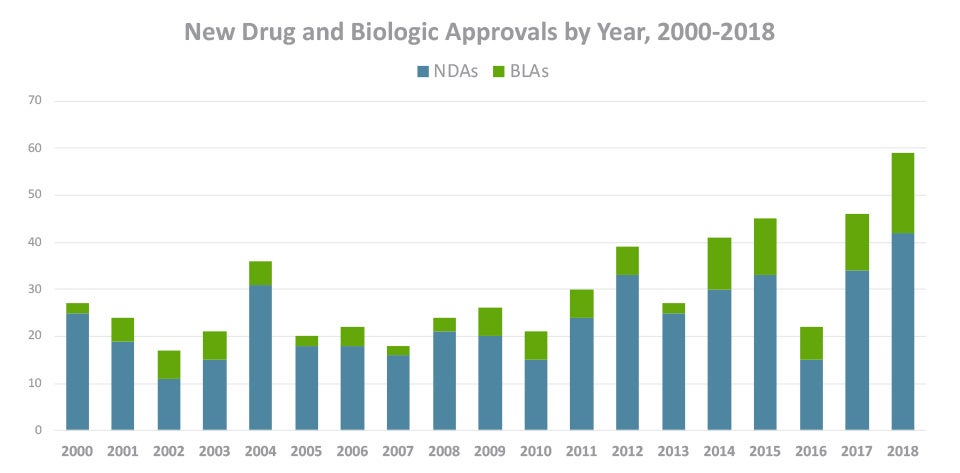
The FDA recently reported that 57 New Drug Applications (NDAs) and biologics license applications (BLAs) were filed in the first quarter of FY 2019, which is higher than any quarter of the previous year. This comes on the heels of a record-breaking 2018, which saw 59 drug and biologic approvals, more than any year in history. As pharmaceutical and biotech companies face increasing pressure to bring more of these medicines to market faster, they’ll look to optimize every step of the regulatory process and they’ll need the right RIM technology in place to support those changes.
Here are three best practices for selecting and implementing a new RIM solution, which will help facilitate system adoption and keep your project on track from kick-off through post-deployment.
1) Leverage established RIM processes and resources wherever possible
First, it’s important to choose a RIM solution that’s preconfigured to support regulatory business processes. That includes features like document templates, which streamline authoring, and boilerplate text, which promotes consistency across applications and submissions. Pre-configured functionality can also speed up legacy document and data migration and help make downstream validation more efficient.
When it comes to implementation, some technology partners will offer a packaged approach that could cut down project timelines from several months to several weeks. Others may recommend a phased approach to get users up and running with a standard configuration and then refine functionality as business processes mature. To determine what path may be best, connect with others that have tackled similar projects. Find out how they determined an implementation strategy and what lessons they learned in retrospect.
2) Consider business and technical needs in parallel
In addition to system configuration, it’s critical to focus on evolving business processes. Set aside time to understand how these processes will improve with a new RIM solution, where new processes need to be defined, and which legacy processes should be retired. Remember that these shifts will also require communication planning, standard operating procedure (SOP) authoring or updates, user account management, and training tailored to each regulatory function.
Ideally, new processes are introduced concurrently with system go-live, so it helps to map out important stages of the implementation process like configuration lock, user authorization testing (UAT), performance qualification (PQ), and accepted validation report.
3) Embrace a hands-on approach
It’s crucial that core users get acclimated to the new system as early as possible. This will help determine if a standard configuration meets company needs, where gaps exist, and how to address them before go-live. Set up in-person workshops and process exercises to test functionality, and if possible, secure access to a separate development environment. Users who are involved in testing will become system experts and champions in the future, so help them gain confidence through hands-on experience.
Having recently completed a Veeva Vault RIM implementation, Michael Monahan, the senior director of regulatory affairs and quality assurance at Crinetics Pharmaceuticals, shared, “Our team was a bit tentative to import documents and make mistakes, but we granted them access to the sandbox and instructed them to play around and get comfortable with the system. That proved to be a huge help down the line.”
To learn more strategies for a successful RIM implementation, watch our on-demand webinar.

