Leading trial master file application
Veeva Vault eTMF is the leading trial master file application used to ensure the quality, timeliness, and completeness of a TMF.
Enterprise content management capabilities
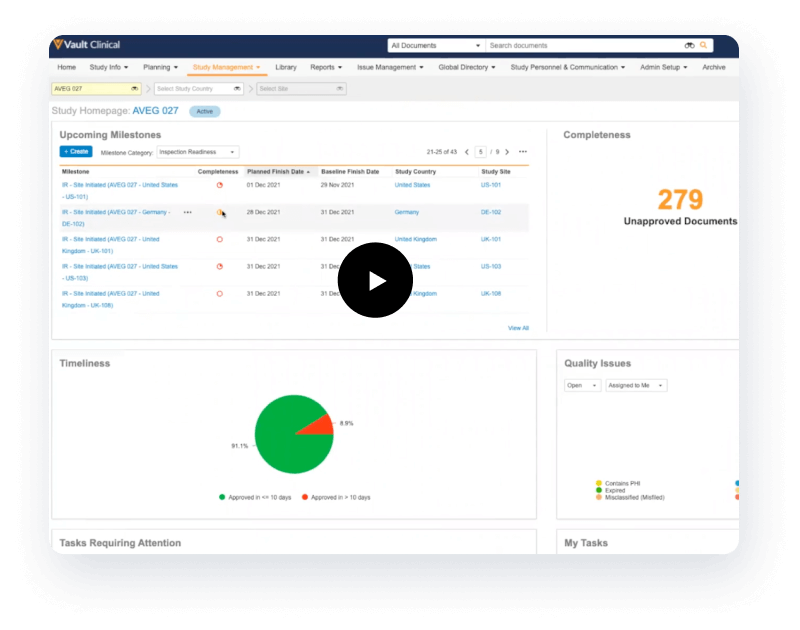
It provides full enterprise content management capabilities for upload, version control, QC and approval, and real-time co-authoring with Microsoft Office for study documents such as consent forms. It’s highly efficient and performant and supports global outsourcing.
Compliance and collaboration workflows
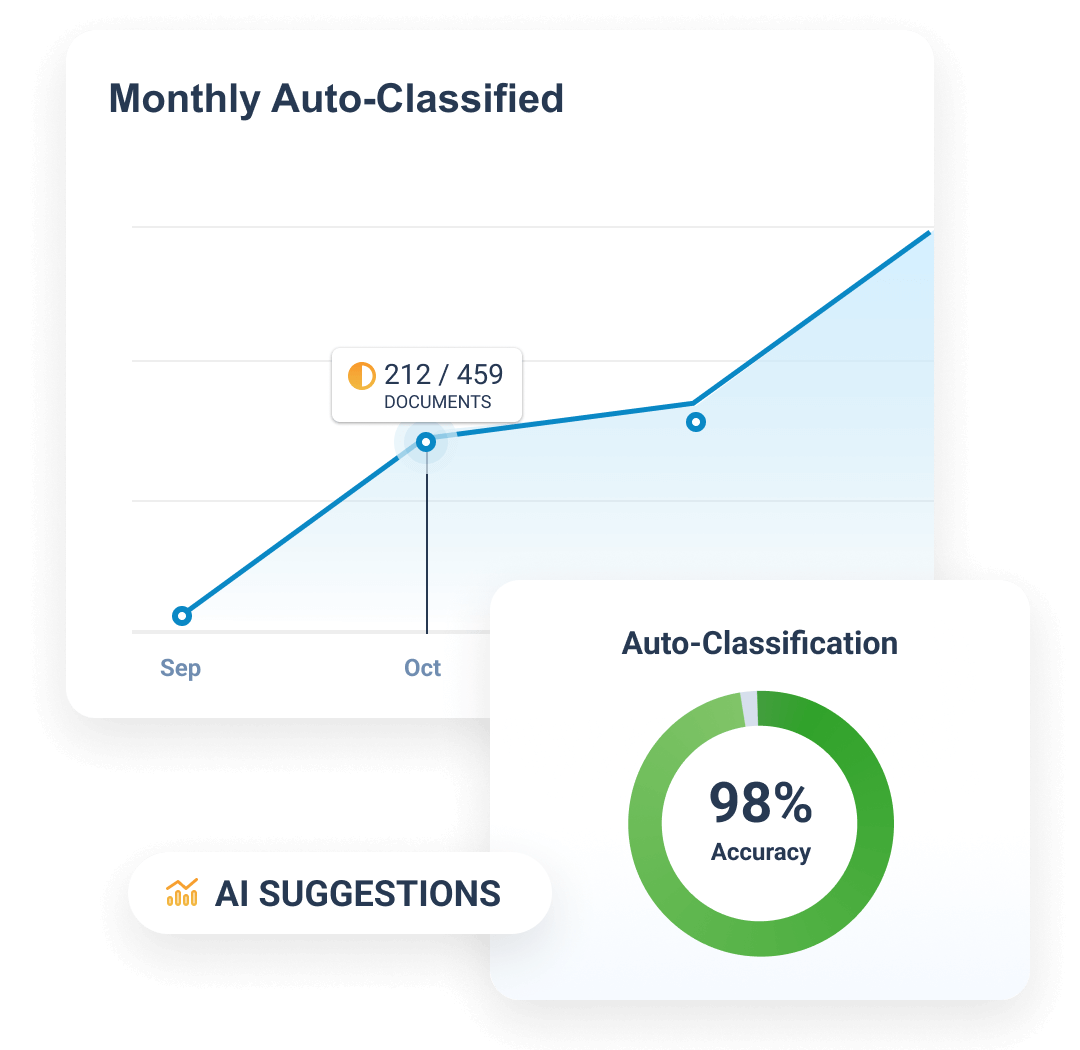
Completeness and timeliness are managed through Expected Document Lists (EDLs). Content files are auto-classified through the TMF Bot, and classified content is matched automatically to EDLs. The TMF Transfer feature simplifies exchange between sponsors and CROs by sending completed TMFs at study close.