Regulatory Transformation
with Unified RIM
End-to-end regulatory information management on a single platform.
Learn about IDMP and best practices from BMS and GSK
Veeva RIM
Veeva RIM unifies regulatory systems and processes on a single cloud platform to enable end-to-end submission and registration management.
RIM applications share a common data model, which allows for regulatory business functions to run in one Vault.
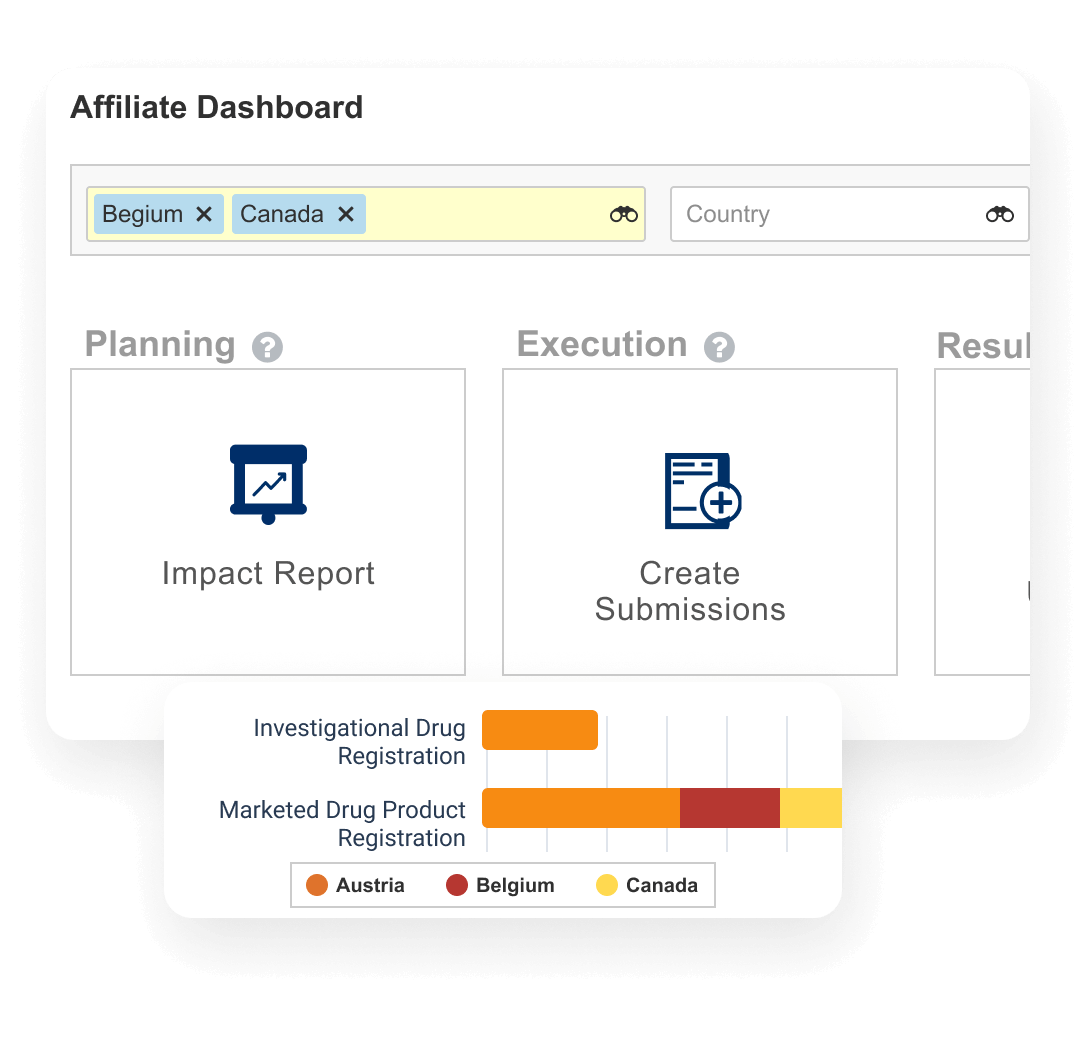
Veeva Registrations
Plans, tracks, and reports on global health authority product registrations and associated changes.
Veeva Submissions
A content management application used to plan, author, review, and approve regulatory submissions.
Veeva Submissions Publishing
Produces compliant published submissions ready to send to global health authorities.
Veeva Submissions Archive
Provides storage, navigation, and search of submitted regulatory applications and related correspondence and questions.
Veeva AI for Regulatory
Health Authority Interactions Agent
Automates HA interactions for faster simultaneous approvals globally.
Application Assistant Agent
Conversational insights into regulatory activities with clear and consistent narratives.
Veeva Connections
Veeva Connections are Veeva-delivered integrations that seamlessly transfer data and documents.
RIM–Clinical Operations Connection
Enables users to automatically share product, study, and site information.
Quality-RIM Connection
Shortens the overall timeline from change control event creation to implementation.
RIM–PromoMats Connection
Integrates compliance package generation for direct publishing to health authorities.
See a full list of available Veeva Connections.
Demo
Veeva RIM End-to-End Platform
Veeva RIM provides an authoritative source for regulatory documents and information globally. Content and data converge in a single cloud platform that unifies registration tracking, correspondence and commitments, submission document management, dossier publishing, and regulatory submission archiving.



