Building A Successful Claims Foundation
With EU MDR in full swing, medical device and diagnostics companies across the globe are revisiting (or should revisit) how they manage claims. For many medtech companies the process is built from a network of disparate solutions supplemented with trade knowledge amongst brand teams.
Unless you’re a member of a specific brand team, it’s often unclear how claims were derived. Not to mention whether the substantiating documents are clear, sufficient, and easy to find. This makes sharing claims, driving brand consistency through re-use, and maintaining compliance, more difficult.
More than 15,000 claims are managed today within Veeva Vault PromoMats, and the number is quickly rising. Volume alone suggests that more formal processes and supporting technology can help. Shifting global regulations and potential for greater speed to market provides incentive to adapt.
“Our review and approval team loves the impact of modernizing our claims approach. They’re able to quickly understand the context within promotional material, view the correct substantiation, and eliminate inefficiencies in their evaluation.” – Montoya Love, Director Regulatory Compliance, BD
Below are observations and best practices for managing claims and how medtech companies can transition to a more modern approach leveraging process improvements and technology.
Why effective claims management matters
Claims play an essential role in the commercialization of products. Any time a statement is made about the efficacy of a device, it must be supported by substantive scientific results and avoid anything potentially misleading to a patient or healthcare professional (HCP). From a regulatory perspective, particular attention is given to the safety and performance of a product with governing authorities advancing strict requirements on clinical support data.
For instance, EU MDR clearly prohibits misleading claims from all forms of product communication and states that “claims must refer to the device’s intended use, safety, and performance”. From a patient and HCP perspective, ensuring we are providing up-to-date and consistent messaging across markets is key to patient understanding and avoiding confusion.
Despite the importance of claims in industry, many organizations have yet to figure out the best pathway to contain and manage claims used in marketing materials. There is a great opportunity to update both systems and processes to help meet the needs of governing bodies, internal stakeholders, and end users in an effort to elevate customer experience.
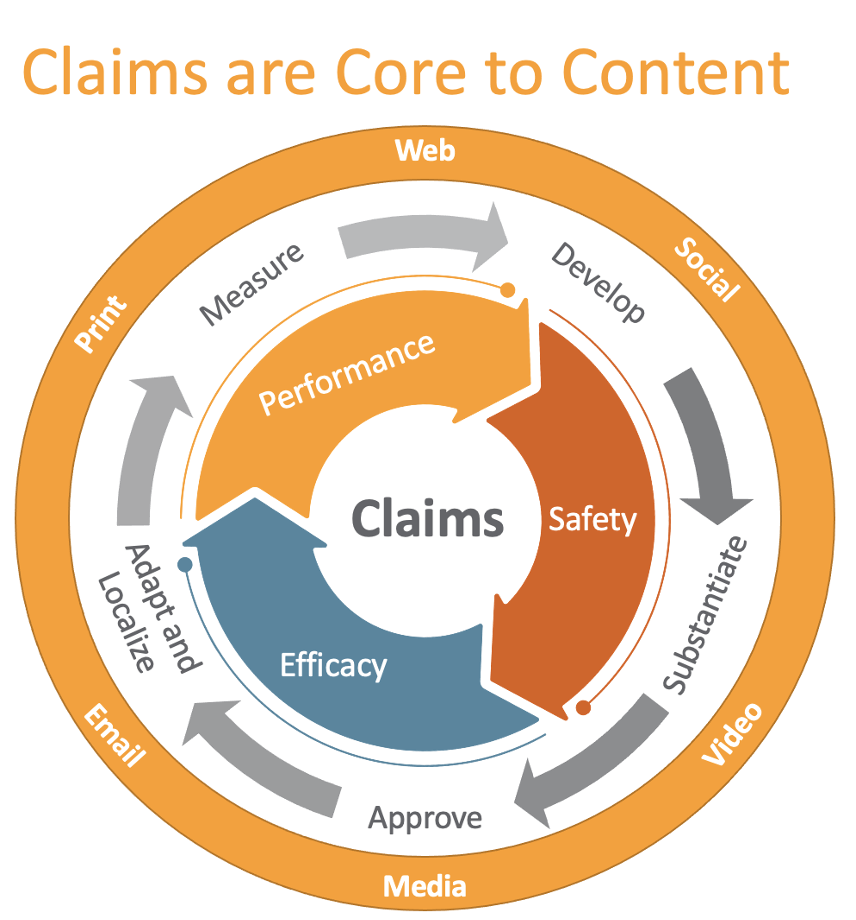
Building a Foundation
Ask most medtech companies how they manage claims today and you’ll get a variety of answers. Perhaps nothing formal is in place, they’re leveraging spreadsheets on local hard drives, or they’re using the marketing materials as a record of approved claims. While these systems may have worked to date, it’s becoming increasingly more difficult to manage as regulations tighten and marketing teams look to drive more consistency across their brand.
Along with these challenges comes great opportunities to improve. In working with our customers, we have observed 3 key elements that are critical to building a strong foundation for claims management.
- Define a common vocabulary – Ensure your definition of claims and surrounding metadata are understood across the organization.
- Push for a unified architecture – Ensure that systems and people are connected and communicating with one another.
- Establish KPIs – Think through the right metrics you’d like to capture, these can be quantitative and/or qualitative. Consider measuring re-use of claims across affiliates and tactics.
As simple as they may seem, each of these activities require strategic thinking, business case planning, and buy-in across the organization. The key to success is to engage with internal stakeholders early, build your business case, and ensure you have executive sponsorship. Starting can feel overwhelming, but the benefits can be significant. Montoya Love, Director Regulatory Compliance at BD has stated “our review and approval team loves the impact of modernizing our claims approach. They’re able to quickly understand the context within promotional material, view the correct substantiation, and eliminate inefficiencies in their evaluation”.
Setting Up for Scale
While modernizing your claims process solves many immediate pain points, it’s also comforting to know that it sets you up for future success as you grow. Establishing a consistent approach to claims is fundamental to enabling more advanced marketing techniques like modular content and omnichannel. These things require moving with speed, while maintaining compliance. An agreed upon framework for claims enables review teams and creators to work more seamlessly together throughout the entire content lifecycle and helps you get your message to patients and HCPs faster and more effectively.
Watch this video featuring Montoya Love from BD for additional perspectives on modernizing claims management. To learn more, please reach out to your Veeva Account Partner or visit veeva.com/medtech/promomats.
Jeff Gorski , Senior Director, Commercial Strategy, Veeva MedTech
Ramona Galantonu, PhD, Director, Commercial Strategy, Veeva MedTech EU