Unify training and qualification management
Announced 2018
Status MATURE
Customers 100+
Veeva Training manages quality training requirements to ensure compliance and role-based qualification for job and audit readiness. With centralized training records and a single audit trail, medtech companies can easily demonstrate compliance – always staying inspection ready.
Veeva Training streamlines training from creation to distribution and completion by seamlessly working with Veeva QMS and Veeva QualityDocs.
Demonstrating compliance is easy with centralized training records and a single audit trail, reducing audit preparation time and always staying inspection-ready.
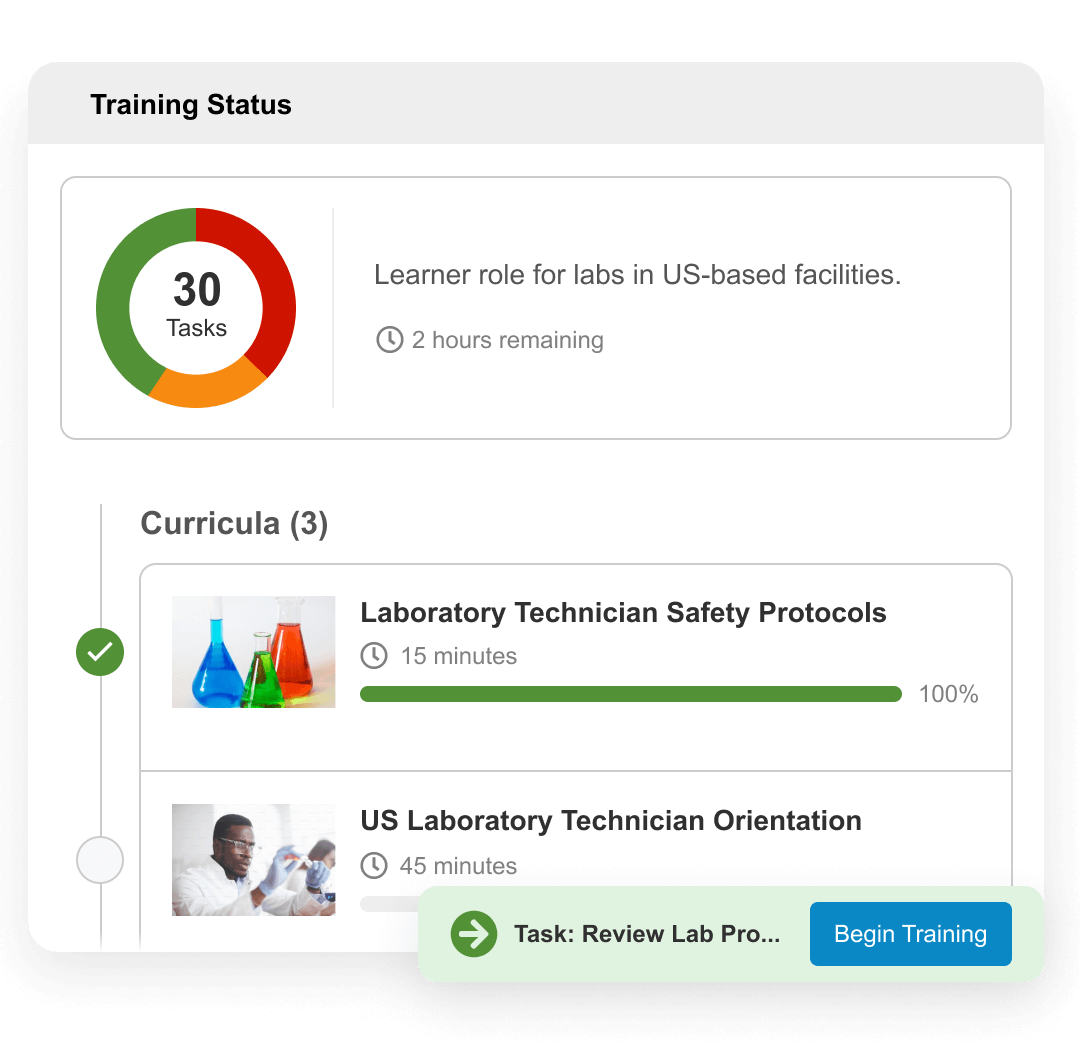
Veeva Training Impact
Qualification management
Manage sophisticated training matrices using flexible, role-based alignment of users and content.Greater training compliance
View upcoming or complete training tasks with an intuitive user experience that makes it easy, even for the occasional user.Unified quality
Seamlessly manage training programs, quality processes, and content, providing a single user experience on a unified cloud platform.Always audit and inspection ready
Easily demonstrate compliance with training reports and centralized, comprehensive training records and activities.
See Veeva Training in action
Customer Success
Medtechs unify quality management with Veeva Quality










View infographic
Insights from industry leaders on how the industry is thinking about proactive quality management
Read article
Philips and Eli Lilly discuss how the role of quality must change and expand

Read article
Bio-Rad, Thermo Fisher Scientific, and the FDA share how global harmonization efforts benefit the industry





