Streamline quality document control and GxP compliance
Announced 2013
Status Very Mature
Customers 100+
Veeva QualityDocs provides a single source for all quality, manufacturing, validation, and other GxP documents and records. The cloud model makes it easy and cost-effective to author, collaboratively review, and approve documents.
QualityDocs reduces the burden of GxP compliance by automating and managing document control processes, helping medtech companies maintain high quality standards at all times.
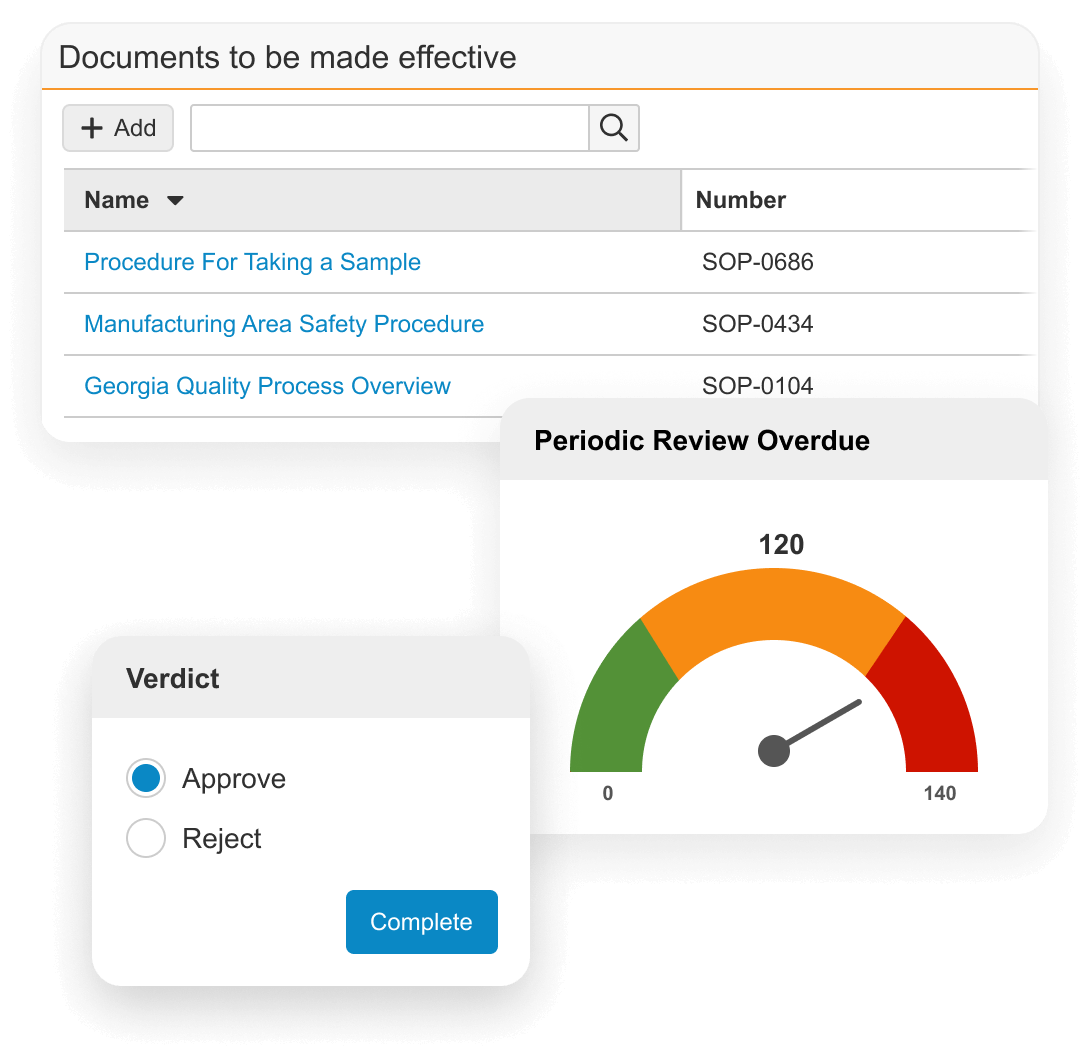
Veeva QualityDocs Impact
Improve quality and compliance
Quickly establish good quality practices and facilitate 21 CFR Part 11, Annex 11, GxP, and other regulatory compliance.Accelerate user adoption
Intuitive experience drives user adoption and efficiency. Easy access anywhere, anytime, and from any device enables a single system of recordStrengthen reporting and metrics
Reporting and metrics identify the state of control, risk, and drive continuous improvement. Robust quality event linkage to content updates and training ensures up-to-date information.
Customer Success
Medtechs unify quality management with Veeva Quality










Watch video
Corza Medical stays audit-ready with Veeva Quality Cloud

Read article
Bio-Rad, Thermo Fisher Scientific, and the FDA share how global harmonization efforts benefit the industry

Read article
Philips and Eli Lilly discuss how the role of quality must change and expand

Learn more
Veeva QualityDocs Features Brief



