Reduce publishing rework and delays
Veeva Submissions Publishing generates electronic submissions for global health authorities. Veeva releases new templates and validation criteria to keep up with evolving regulations.
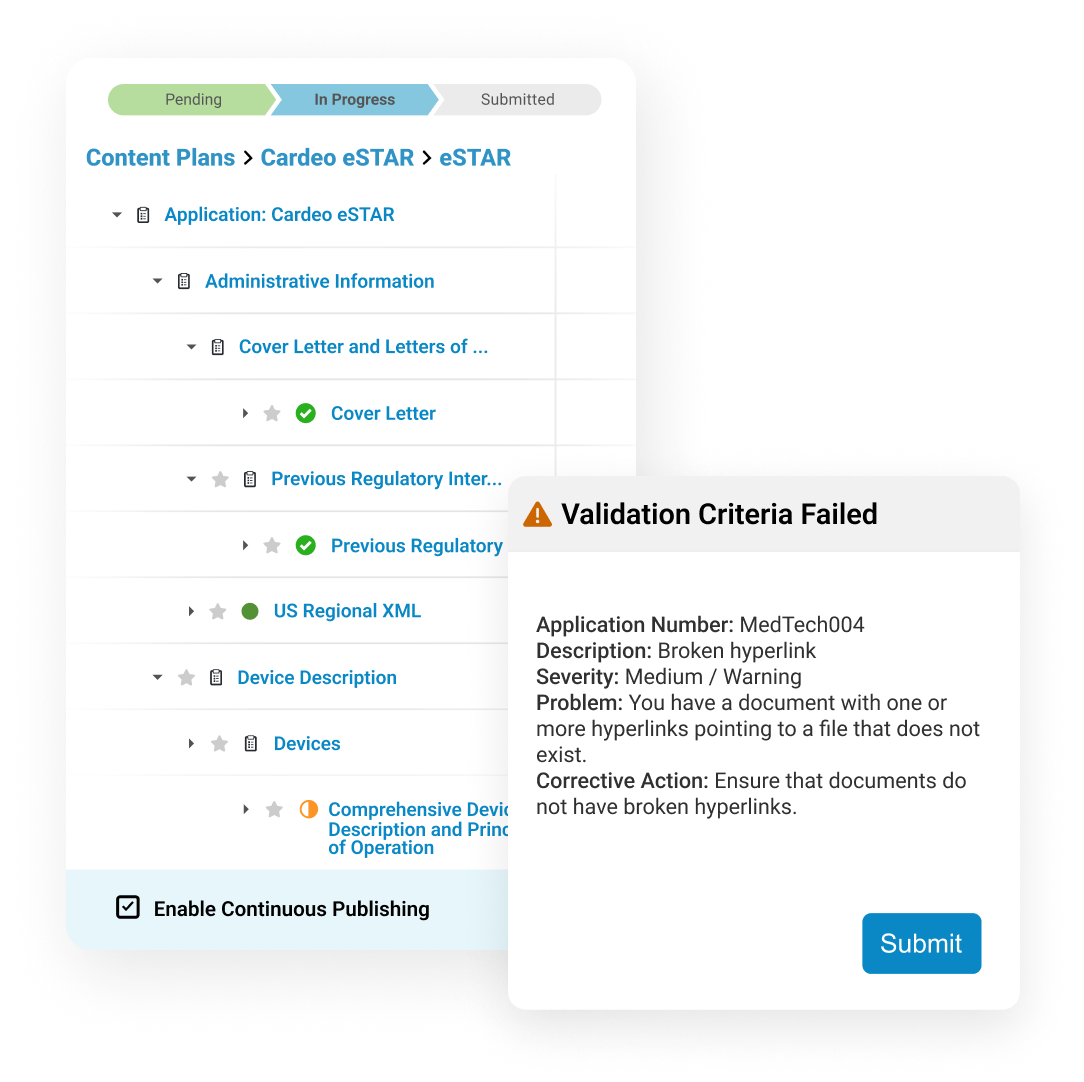
Submissions Publishing leverages content plans created earlier in the lifecycle to start the publishing process as soon as individual documents are finalized.
Users can create internal and external hyperlinks to connect references in the text. They publish submissions directly to health authorities, in markets where allowed.
Dashboards and reports allow publishers to track each submission component as it progresses from authoring to completion.
80%
submission tasks eliminated or simplified
200+
hours saved on approval and renewal reporting
30%
less effort for submission publishing to local affiliates
Veeva Submissions Publishing Impact
Gain comprehensive oversight
Manage end-to-end submission development within a single system.Reduce errors and duplication
Always use the correct document versions with no manual tracking or transfers.Improve efficiency
Complete publishing steps earlier in the cycle during submission authoring and approvals.
See Veeva Submissions Publishing in action
Customer Success
Medtechs ensure compliance with Veeva RIM





Read article
Learn how an in-house publishing model can drive greater efficiency, more flexibility, and improved compliance

Read white paper
See how six leading GenAI models stack up against human experts on typical regulatory affairs tasks

Read white paper
Leading medtechs use RIM solutions to scale efforts, accelerate timelines, and maintain compliance

Learn more
Veeva Submissions Publishing Features Brief


