Streamline global regulatory compliance and information management
Globalization, supply chain complexity, patient safety, and new regulations are significantly impacting the total product lifecycle. Managing global compliance can involve more than 100 different systems and spreadsheets, often resulting in manual, cumbersome processes and a slower time to market.
Veeva RIM enables medtech companies to unify systems with a single source of truth for registrations, submissions, publishing, and archival to ensure global regulatory compliance and speed to market. A real-time regulatory information feed, through our partnership with leading analytics provider Redica, turns data into actionable regulatory intelligence in the context of your portfolio.
-
Gain Real-time Visibility
Facilitate better access, visibility, and control over regulatory documents, data, and processes in real-time.
-
Track Global Registrations
Track and understand the status of your registrations across the globe and view KPIs in real-time to stay proactive.
-
Ensure Compliance
Keep up with a growing amount of information, adapt to the pace of change, and collaborate across the organization.
-
Manage End-to-End Submissions
Author, plan, collect, and approve documents for submission to regulatory authorities in industry standard and market specific formats.
Products
Veeva RIM
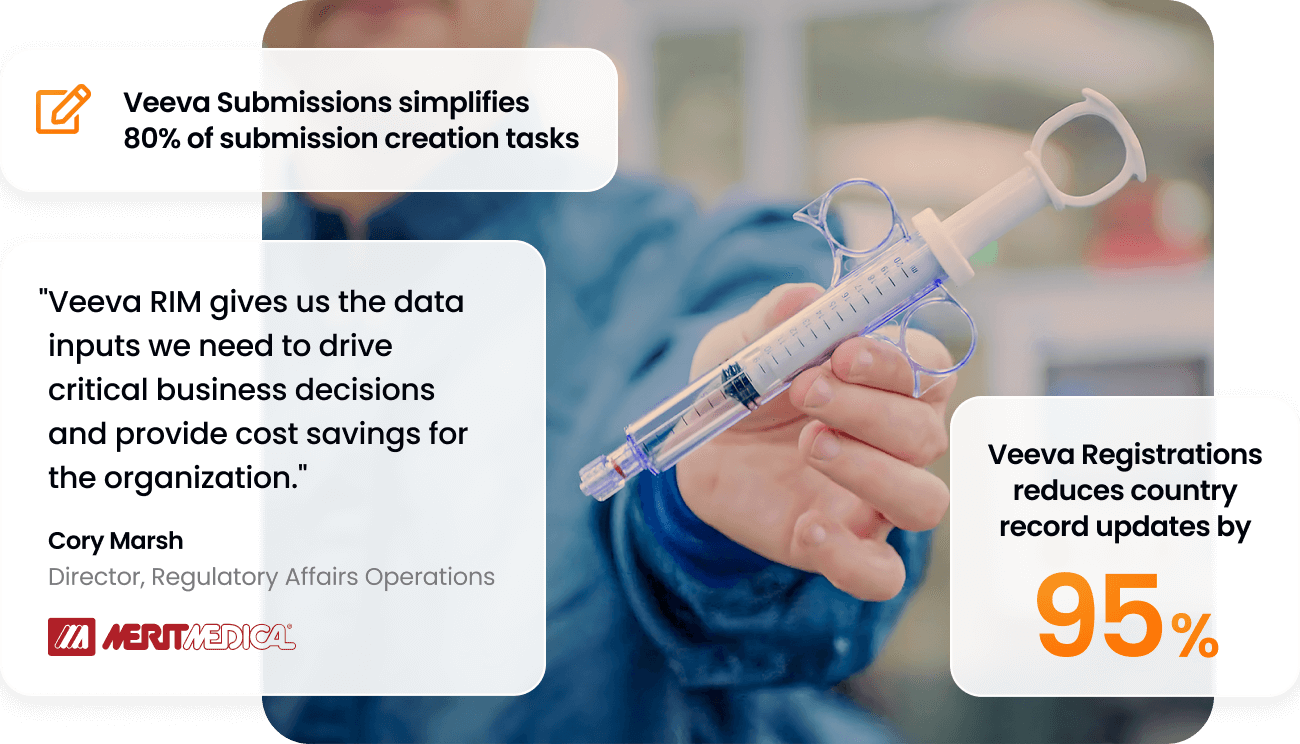
Veeva RIM is a unified group of cloud software applications that provides end-to-end visibility, oversight, and control for all regulatory compliance documents, data, and health authority correspondence, throughout the total product development lifecycle.

Veeva Registrations
Track product registration data, timelines, and the regulatory impact of product changes.
Veeva Submissions
Plan, author, review, and approve submissions.
Veeva Submissions Publishing
Hyperlink and validate behind-the-scenes for greater automation, transparency, and speed.
Veeva Submissions Archive
Store your complete history of regulatory submissions in the cloud.
80%
of submission creation tasks eliminated or simplified
200+
hours saved on product approval and renewal reporting
95%
less time to update country records
Read article
How Alcon and Merit Medical are creating regulatory and quality business value

Read industry report
Data and insights on data quality and efficiency from medtech regulatory leaders
Read article
How Cook Medical is empowering change champions for a global RIM rollout

Learn more
Veeva RIM Features Brief

View eBook
How to find the right RIM solution to meet your team's needs

Read white paper
Electronic Submissions are the Future of Medical Device Regulation – Why the Wait?

Read study
How to evaluate AI's capabilities and potential in medtech regulatory affairs

Read white paper
How to tackle complex medtech regulations with existing resources

