Standardize global quality processes
Announced 2016
Status Very Mature
Customers 100+
Veeva QMS provides a unified global quality management system – for all parties – enabling end-to-end process control and visibility for medtech quality teams.
Easily support proactive management initiatives and manage non-conformances, CAPAs, audits, complaints, supplier management, field actions, and change control processes, or configure your own.
Take a holistic approach to view, understand, and manage quality risks and issues to improve decision making.
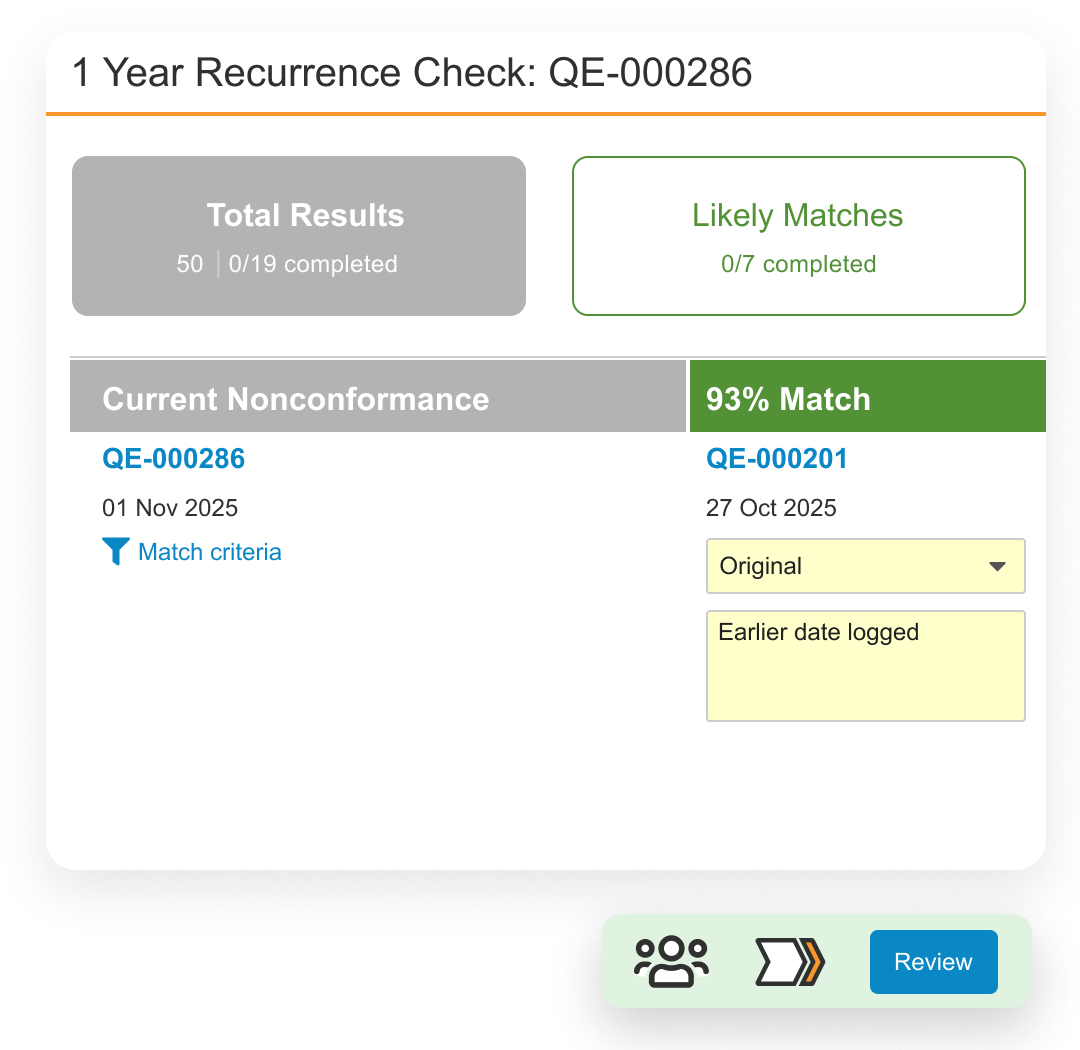
Veeva QMS Impact
Global alignment with partners
Easily incorporate all parties (sites, suppliers, and contract manufacturers, labs, and research organizations) for continuous quality process management, driving and end-to-end control.Complete visibility into quality processes
Track quality processes proactively with the right level of visibility to support timely decisions and accelerate cycle times.Greater compliance
Ensure global compliance with seamlessly connected and controlled processes that are part of the Vault Platform of applications.Rapid time-to-value
Leverage a ready-to-use, scalable application with built-in success practices and automated workflows to improve operational efficiency in record time.
See Veeva QMS in action
Customer Success
Medtechs unify quality management with Veeva Quality










Read article
Thermo Fisher Scientific shares insights from its multi-year effort to instill a culture of quality

View infographic
Insights from industry leaders on how the industry is thinking about proactive quality management
Watch video
Philips modernizes QMS approach to manage risk

Read Brief
Veeva QMS Features Brief



