Edwards Lifesciences: Five Enterprise-wide eTMF Change Management Techniques
How Edwards Lifesciences developed a multi-part structure to facilitate successful eTMF adoption, collaboration, and management
1. Establish a governance board
Edwards’ TMF Governance Board is a core component of the company’s TMF strategy. Developed by representatives across different functions, and the company’s clinical IT partners, the board harmonizes best practices for managing the eTMF and creates a structured mode of communication across business units. Not only does the board ensure effective change management for system and process updates, it also facilitates communication up, down, and out to the rest of the company’s TMF user communities. It also creates visibility, which is key to change management, says DeJesus. “The governance board allows business unit representatives to hear about updates firsthand and communicate them to the rest of their teams.” During biweekly meetings, board members discuss upcoming internal and external audits, review open issues, and collaborate on processes and templates. They also discuss and approve change requests. “We make sure that the eTMF we share continues to function well for all the business units, so that every business unit’s needs are considered,” DeJesus says.2. Share the work and establish accountability across functions
While conducting an internal audit in advance of an inspection, DeJesus discovered that the clinical operations team had to spend a substantial amount of time locating documents. She also found that the latest version of a document that the auditor had requested had not been uploaded to the eTMF. The root cause of the problem was the fact that the clinical operations team was handling all the documentation processes. To improve TMF training and accountability, Edwards launched OneTMF, an initiative that restructured the way users upload and store documents. Instead of putting the entire burden on the clinical operations team, designated SMEs from each functional group (core labs, data management, statistics, safety, etc.) routed documents on their own. “Designating SMEs to share the work eliminated missing documents, improper versions of documents, or storing documents outside of the TMF,” DeJesus said. “We now have a level of accountability within each functional group that we’ve never had before.” Edwards implemented OneTMF in four phases, beginning with the formation of a OneTMF project team and continuing with executive buy-in, concept approval, implementation and roll-out. In 2021, Edwards celebrated the launch of OneTMF with a social event. The company is currently working with the functional groups to reinforce concepts and fine-tune the program.3. Appoint SMEs within the global user community
The company appointed SMEs based in locations worldwide to take responsibility for Veeva Vault and TMF in general. These SMEs reinforce communication distributed from the TMF governance board. They also facilitate peer-to-peer communication to escalate any issues that the functional team encounters. By designating SMEs in multiple parts of the world, Edwards enabled TMF users to get their questions answered quickly by local experts, without having to wait for a user in another time zone to get to the office.4. Develop a TMF Index to provide easy access to guidance
Edwards also established a TMF Index based on the DIA TMF Reference Model, which provides standardized taxonomy and metadata and outlines a reference definition of TMF content.1 The TMF Index helps ensure consistency and quality. It also includes a reference guide to help study teams use the template. “All the guidance we need is spelled out in the TMF Index,” DeJesus said. With minor adjustments, teams use the template for every study.5. Organize user summits
To further broaden its communication channels, Edwards launched an annual TMF Summit. Now in its fourth year, the event includes panel discussions, presentations, and breakout sessions designed for Edwards TMF users. Its first year, the event drew 100 employees. Last year, about 300 attended remotely from offices around the world. Edwards targets sessions for both beginner and advanced eTMF users, while presentations center on everything from site binder review to quality checks. “Our corporate compliance group even joined to share how working with eTMF has changed its internal auditing process,” DeJesus reports.An additional post-COVID 19 bonus: Introduce remote and hybrid roles
After launching the TMF Governance Board, OneTMF, and other initiatives, Edwards had to adjust its strategy to account for the shift to remote work brought about by the pandemic. That included helping employees adjust to remote work environments as well as supporting clinical operations and study teams as they adapted to remote monitoring, digital source data collection, and remote source data verification. All these changes brought a need for more flexibility in an unpredictable environment. “We had to realize that many of our sites were short-staffed, as clinicians shifted from research to active frontline healthcare, ” DeJesus says. “We needed to remain sensitive to their needs as we emerged from the crisis.” Moving forward, Edwards will continue to reconfigure its eTMF every few years and incorporate new tools at its own pace. As it does, the company has established a foundation for ensuring seamless technology adoption. These efforts are helping bring the company closer to its end goal: compliant clinical trials designed to improve heart health for patients worldwide.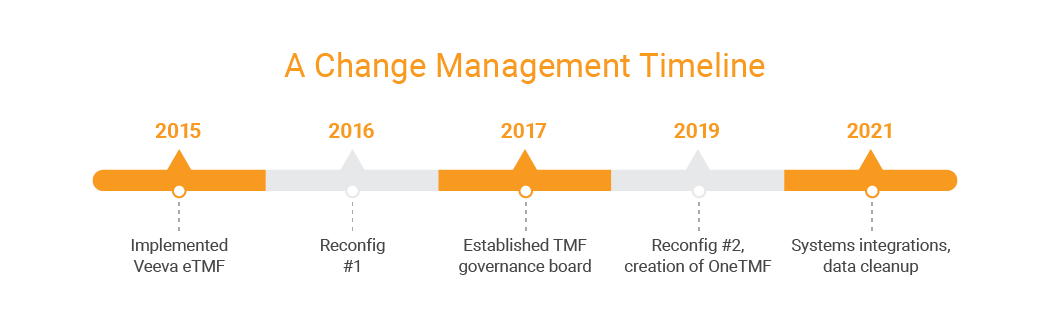
Hear Brenda deJesus on how her team at Edwards Lifesciences stays inspection-ready with Vault eTMF..
*Rupani ZM. “Clinical Trial Master File Migration: A Preordained Step for a Centralized Electronic Trial Master File,” Perspect Clin Res. 11(4) 2020;139-143.**Veeva Systems, “Edwards lifesciences: Best Practices for Enterprise-Wide TMF Management and Collaboration”