Modernizing Clinical Trials: Keeping Pace with Medical Device & Diagnostics Innovation
Clinical studies are the foundation of the successful development of medical devices and diagnostics. They are also complex – from site selection to subject enrollment, study monitoring , and management of the resulting data.
This year, on top of the typical challenges, the coronavirus brought many clinical studies to a slowdown or a standstill. With the reduction of traditional face-to-face interactions at the site level, the pandemic has been a significant roadblock for the clinical research community.
Challenges bring innovation, and forward-thinking companies learn to adjust to the times. Many are now moving forward with a hybrid of tried-and-true methods and new remote management strategies to move clinical research into the digital realm.
“With the reduction of traditional face-to- face interactions at the site level, the pandemic has been a significant roadblock for the clinical trial community.”
Against this backdrop, optimism is rising for clinical development in the remainder of 2020. An audience poll during a recent Veeva Systems webinar found that 54% of device and diagnostics industry professionals anticipate a full recovery in clinical study activity will come before the end of the year.
There has been an initial uptick in activity heading into June, as some studies are resuming, and elective medical procedures are starting again at a state level, says Kevin Liang, Senior Director of Clinical Strategy for Veeva Medical Device & Diagnostics.
Modernizing your clinical trials
During the webinar, Dr. Kendra Hileman, Vice President, Head of Clinical R&D for eye care company Alcon, noted that her company is taking steps to modernize its clinical research program with remote monitoring, virtual visits and the implementation of technologies to support efficient clinical operations at all levels including trial master file (TMF), study and data management.
Alcon has taken some of these steps by leveraging Veeva’sVault Clinical Suite, as well as its quality documents and regulatory information management solution, to modernize its operations. Now, Hileman said, all of the company’s trial systems can work together and share data.
The modernized approach enables Alcon to streamline end-to-end processes, and improve how sponsors, CROs and sites work together by digitally managing electronic data capture and management, eTMF, clinical study management and payments.
These types of improvements are high priorities for clinical research development. An audience poll found priority areas for technology upgrades include:
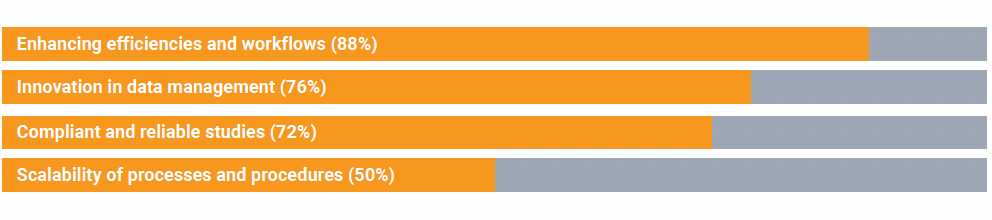

Matthew Purner, Senior Director of Clinical Affairs for genomics leader Illumina, highlights that his company manages electronic data capture of “huge sequencing files” and anything that can improve the management of the data is a high priority.
In the coming years, he predicts another significant area of focus will be the ability to connect with hospital systems and EMRs, as well as having EMRs and case report forms populate automatically based on connections with other systems. This way, testing outcomes can follow patients as they come in for treatment with the ability to quickly identify patients and match them to clinical trials.
Going remote
David Templeton, Manager of Clinical Systems for medical device manufacturer Penumbra, says part of his company’s clinical trial modernization has included reducing the number of onsite visits, going remote more often for qualification and initiation visits, and conducting closeout visits on a fully remote basis.
Penumbra also sees opportunities in the areas of electronic consent, remote training on use of devices and remote monitoring. Leveraging its clinical systems to impart both internal efficiencies and site collaborations always has been high priority for Penumbra.
From a systems perspective, Templeton prioritizes tight integration of systems, as well as reduction of the number of platforms and systems being used. As companies move toward more remote trial operations, it will be essential to leverage better tools that are more powerful and easy to use for reporting, he notes.
“Our company’s clinical trial modernization has included reducing the number of onsite visits, going remote more often for qualification and initiation visits, and conducting closeout visits on a fully remote basis.” — David Templeton, Manager of Clinical Systems, Penumbra
Learning from COVID-19
The current pandemic has highlighted various opportunities, as well as gaps, in the clinical research landscape, “and it would be foolish if we did not take the opportunity to learn and adapt,” says Jennifer Kerr, President of Cook Research. “It’s important to keep simplifying as much as we can, not only for for us as sponsors, but for all of the stakeholders we will interact with in the clinical research.”
Another audience poll revealed the top ways the industry can better support and modernize its effectiveness with clinical study sites and investigators, including:
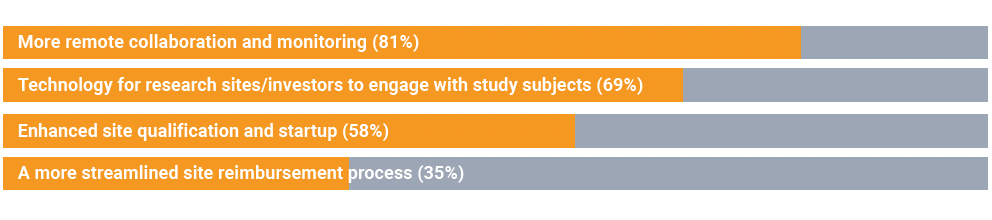
Many of these modernizations can be achieved more quickly and efficiently by partnering with an outside organization that specializes in technology to streamline clinical trial management.
Kerr notes the medical device industry shares common regulations and guidance with the life science and pharmaceutical industries, however, differences do exist in the design and management of clinical studies. Having a partner that wants to understand and support the medical device industry can make the difference in the adoption of new, cost effective solutions within an organization.
With lessons learned and the right tools in hand, medical device and diagnostics companies can emerge better equipped to conduct efficient, compliant and better-informed clinical studies.
To learn more about the modernization of clinical trials, visit Veeva MedTech Clinical Suite.