Modernizing Regulatory Affairs: Veeva MedTech Regulatory Benchmark Study

The past decade has brought unprecedented change to the MedTech industry. New complexities and challenges have come in the form of new regulatory requirements, fierce competition, and rising compliance costs. The European Medical Device Regulation (MDR), for example, emphasizes transparency and provides a harmonized regulatory framework to ensure device safety and performance. However, experts predict that approx. 30% of companies may go out of business, struggling to fulfill the requirements. To gain market advantage, medical device companies need to embrace process harmonization and standardization, pursuing greater regulatory affairs integration across the total product lifecycle. While there are positive signs that many companies are moving in this direction, those that are held back by manual processes and siloed operations stand to hinder compliance and speed to market. Regulatory Pulse Benchmark Study findings From March to May 2021, Veeva MedTech conducted interviews with 15 senior regulatory leaders at major medical device companies to evaluate their regulatory operations and where they stood on the road to modernization. Throughout the interviews, we learned that the regulatory affairs function is ready for a transformation. Many organizations have initiatives in place to harmonize the regulatory process across divisions and geographies where they have typically been siloed. Semi-structured interviews analyzed the state of business in regulatory operations, assessing global compliance and visibility, speed to market, and post-market compliance. The results yield interesting insights:
- 71% of participants – create submission content in siloed, regional document management systems.
- 57% of participants – create reports manually to track performance and KPIs.
- 57% of participants – note inconsistent or disorganized data collection when planning and tracking global registrations.
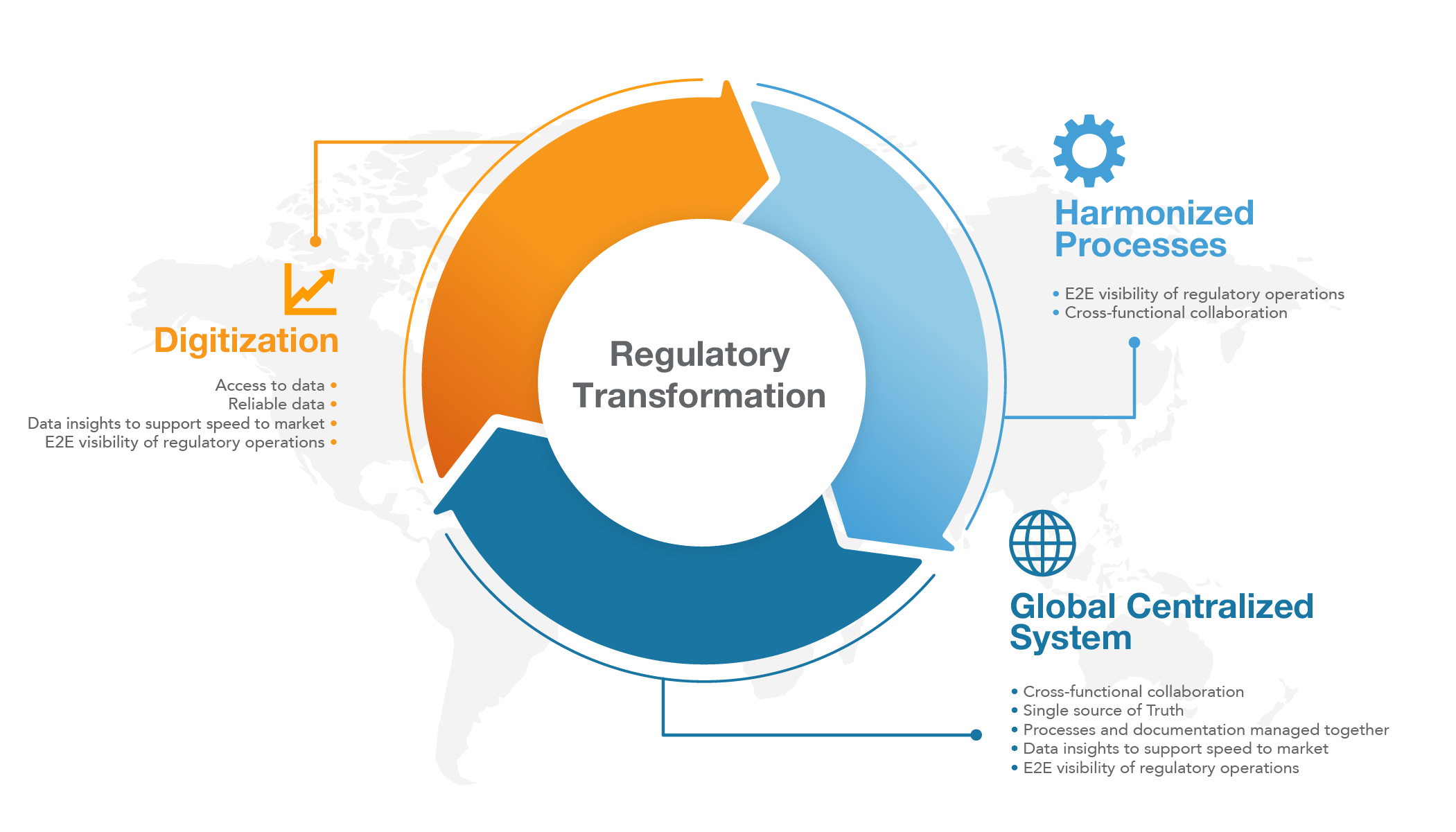
When asked in which regulatory areas there was a need for transformation or modernization, 100% of those interviewed said digitization, the need for harmonized processes, and the use of a global, centralized system that enables cross-functional collaboration. The rationale behind these varied across organizations; however, access to data that results in actionable insights was common among all participants. Connecting data to enable actionable insights Each medical device manufacturer holds a wealth of data when bringing products to market. However, manual processes and siloed operations can obstruct data access and tamper with its reliability, causing difficulties when sharing and analyzing results. The more reliable regulatory data is, the more insights organizations can glean from it, enabling a product to reach the market faster. When regulatory affairs know early on which information is needed for commercialization in the targeted countries, the sooner they can start collecting the data. Speed to market results in faster revenue generation and greater competitive advantage. Having a centralized system in place increases the end-to-end visibility of regulatory operations. It also advances the ability to respond quickly to internal and external events that impact submission documentation and registrations. Enabling fast response and cross-functional collaboration through a centralized system

The ability to quickly respond to changes increases when cross-functional collaboration between regulatory, quality, R&D, and other functional teams is enabled through a centralized system. It allows regulatory affairs to be proactive rather than reactive, enabling teams to focus on value-add activities rather than administrative tasks. The centralized system also functions as a single source of truth to support consistent use and re-use of content, facilitating efficiency gains and reducing compliance risks. Currently, only 7% of those interviewed create, store and manage content electronically across submissions. With increased scrutiny from regulatory authorities regarding the consistent use of content and information across documentation, reports, and other assets, having a single source of truth is vital for medtech organizations across all functions. Turning regulatory vision into reality To ensure compliance and speed to market, regulatory needs to modernize. It’s crucial to have processes and documentation managed together, enabling visibility into market approvals and submission changes throughout the total product development lifecycle. To turn this vision into reality, most regulatory leaders have put things in motion in their respective organizations. There are two regulatory modernization models we identified during the study:
- Regulatory Modernization Model 1
Organizations have defined an organization-wide digitization vision in which regulatory transformation is one of the first initiatives being implemented. The foundation for processes and governance in these organizations is in place, and Regulatory Information Management (RIM) technologies are being evaluated or already implemented. - Regulatory Modernization Model 2
Organizations have not yet embraced an organization-wide approach to digitization. However, regulatory leaders see the urgent need for change and are proactively working on process harmonization and standardization.
They build the foundations, including people, processes, and technology requirements, to be prepared for digitization and technology selection. This proactive approach allows regulatory affairs to be at the forefront when an organization-wide digital transformation strategy is rolled out.
Regulatory affairs is a critical function throughout the product lifecycle. From leading the premarket strategy during the R&D phases to drafting regulatory submissions and post-market surveillance, this function ensures continuous compliance and has an increased impact on business decisions. The regulatory function is often seen as a barrier to market. However, as the pace of change and innovation accelerates, organizations need to recognize the pivotal role of regulatory affairs in getting products to market faster. Providing visibility and clarity into the development processes enables regulatory affairs to understand the commercial strategy, product distribution, and business strategy, ensuring compliance and speed to market early on.
To learn more about how medtech leaders are tackling increasingly complex regulations with existing resources, read this white paper.