
Radiometer shares how unifying clinical with Veeva eTMF and CTMS improved collaboration to scale study volume by 400%
View Customer Story

Cochlear improves study quality, compliance, and operational excellence with Veeva eTMF, CTMS and EDC
View Customer Story

Terumo drives global collaboration and maintains audit readiness with Veeva eTMF and CTMS
View Customer Story
Faster, higher quality trials
Veeva CTMS is an enterprise trial management system that provides end-to-end study management and monitoring capabilities for insourced and outsourced trials.
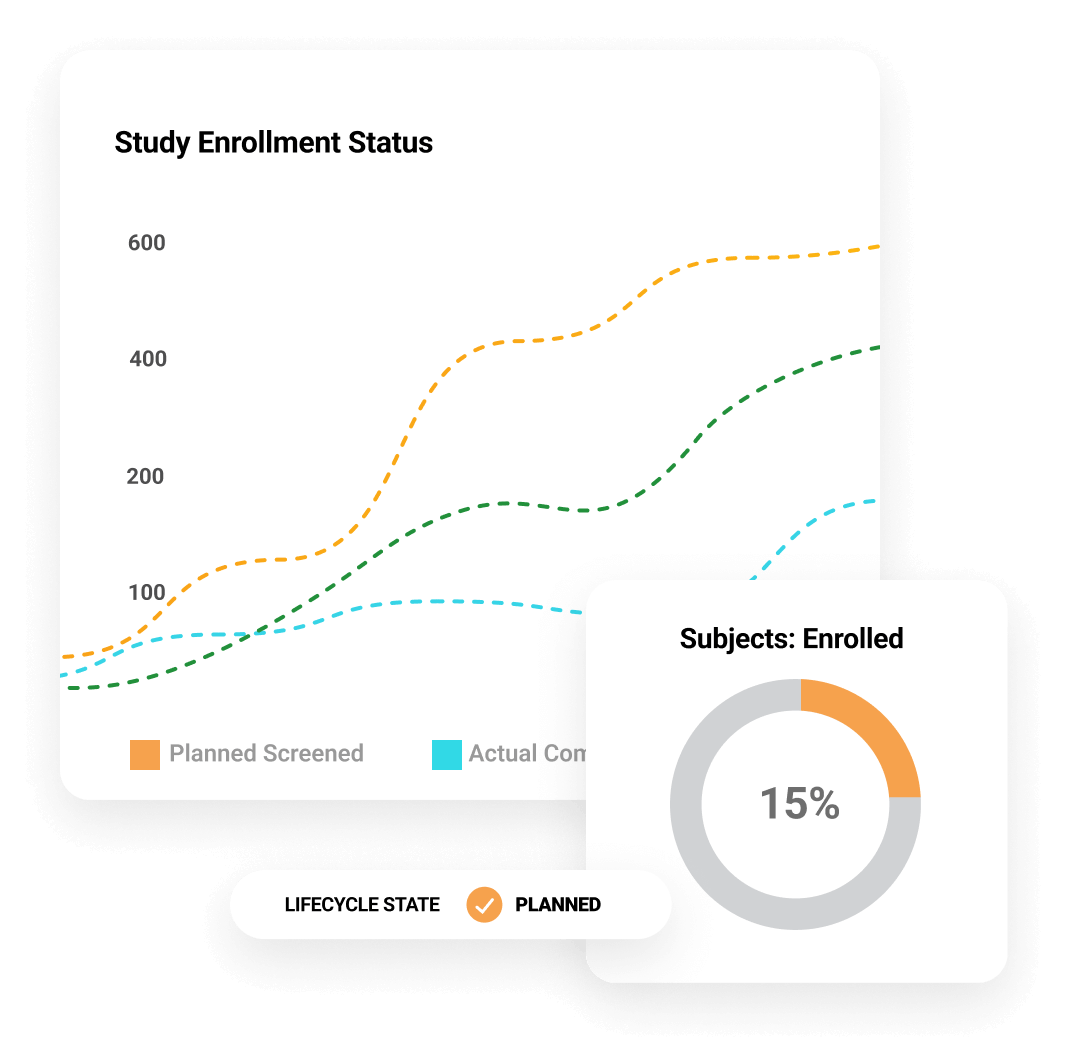
Dashboards and reports help teams track key indicators, including enrollment and milestones, with drill-downs to take action. Monitoring visit reports support automation and dynamic question branching. Trip reports are automatically filed within Veeva eTMF. Issues and protocol deviations are logged as needed and routed through resolution workflows to ensure closure.
Veeva CTMS is connected with Veeva EDC to support enrollment, monitoring, payments, and navigation to casebooks directly from within Veeva CTMS. Connectivity with Veeva Payments streamlines on-time and accurate payment to sites.
30%
reduction in monitoring planning & execution
25%
improvement in issue management efficiency
50%
less time to author visit reports
Veeva CTMS Impact
Speed trial execution
Proactively identify and manage risks to mitigate timeline slippages.Enhance productivity
Equip study teams with role-based dashboards and intuitive navigation.Improve decision-making
Enable closed-loop issue management and strategic planning with a real-time view of trial status.Drive sponsor-CRO collaboration
Monitor portfolio performance to drive impactful discussions and enable proactive actions.
See Veeva CTMS in action
Customer Success
Medtechs speed trials with Veeva Clinical Operations






























Watch video
Radiometer shares how unifying clinical with Veeva eTMF and CTMS improved collaboration to scale study volume 400%

Watch video
Cochlear improves study quality, compliance, and operational excellence with Veea eTMF, CTMS and EDC

Watch video
Terumo drives global collaboration and maintains audit readiness with Veeva eTMF and CTMS help them

Download infographic
The MedTech Clinical Benchmark examines how companies are increasing trial activity to maintain product marketability

Watch video
Integra unifies clinical data and clinical operations to establish repeatable processes and data visibility

Read customer story
NuVasive automates processes and consolidates qualification and GCP documentatios to enable scalability and compliance






