Radiometer Scales Clinical Trials with Unified Clinical Platform
Automated clinical processes help achieve 400% increase in clinical trial activity
Radiometer Scales Clinical Trials with Unified Clinical Platform
As the In Vitro Diagnostic Regulation (IVDR) transition period for high-risk devices ends in May 2025, in vitro diagnostic (IVD) organizations are preparing to comply with stricter standards and more robust post-market surveillance requirements. “IVDR is a challenging regulatory framework. But, it is also an opportunity to innovate and strengthen our commitment to higher quality and patient safety,” says Kevin Valle Lozano, senior clinical project manager at Radiometer.
Headquartered in Denmark, Radiometer specializes in blood sampling and gas analysis. “To comply with IVDR, we needed to conduct bigger, more complex clinical studies,” Lozano explains. In just a few years, Radiometer grew from an average of 5-6 clinical studies per year to managing over 20 clinical studies in 2025. Lozano knew his team needed robust technology and change management strategy to support their growth. They also needed to establish a hybrid operating model. “There is no way that an organization can grow that fast in terms of resources and clinical processes. We had never worked with CROs before, but we had to start outsourcing some activities in order to scale” he says.
In 2023, Radiometer partnered with Veeva Business Consulting to implement a unified digital platform with Veeva eTMF and CTMS. Before implementation, the Radiometer team conducted an extensive user satisfaction survey that guided their change management strategy. “Change management starts before you initiate a program. It requires educating the leadership team and the end-users of the system. It is important to put a line item in the budget for change management,” says Lozano.
“Change management starts before you initiate a program. It requires educating the leadership team and the end-users of the system. It is important to put a line item in the budget for change management.” –Kevin Valle Lozano, Sr. Clinical Project Manager, Radiometer
Assessing gaps with a user satisfaction survey
Before starting its clinical transformation journey, Radiometer did not have a dedicated eTMF. Instead, the team used their DHF to house some study documentation. “Our DHF was very cumbersome to use, so many of our clinical study managers were using interim solutions like spreadsheets and personal documents. Implementing a digital system would help increase compliance,” Lozano remembers.
Radiometer also used paper-based processes to manage trials. “For protocol deviations, we were always printing, scanning, and trying to store all our documents,” says Lozano. “We had data coming in from many different sources, which we tracked manually in spreadsheets. None of our processes were integrated.” Lozano estimates that it took his team about three hours each time to update the information for a clinical study in their portfolio.
Lozano knew he needed to influence change and collaboratively built a business case to update Radiometer’s clinical systems. To identify the gaps in existing processes, Lozano conducted a clinical operations team user satisfaction survey in partnership with Veeva Business Consulting. The results created a baseline of the quality, efficiency, and improved compliance of their current system.
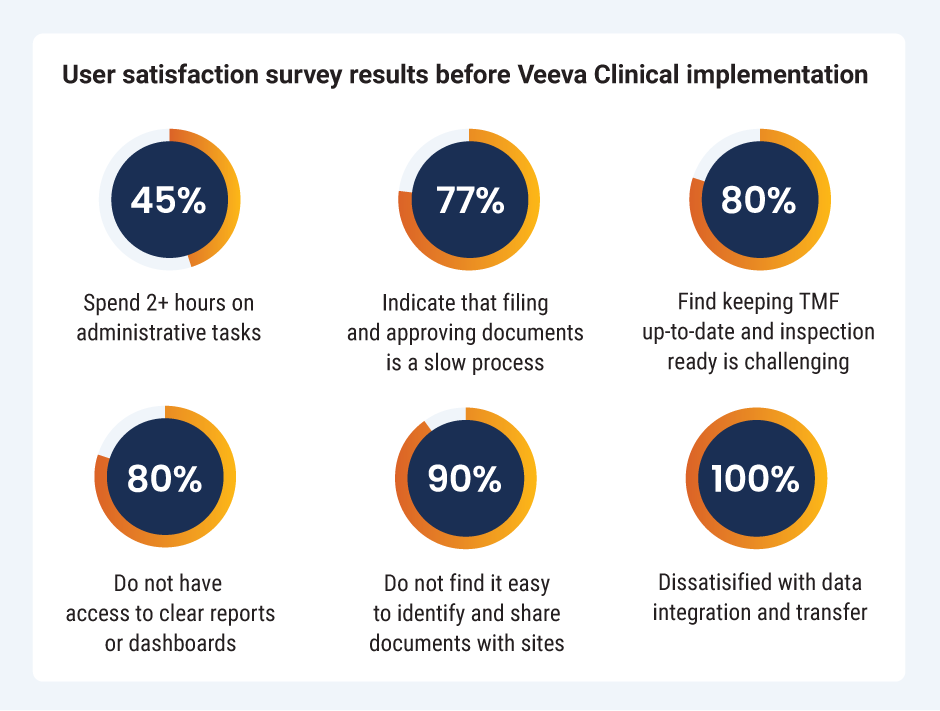
The results were concerning to Lozano, but crucial to creating a strong business case for Radiometer to invest in updated systems. “We conducted a thorough and honest survey that showed the dissatisfaction with our current processes. It was a difficult truth to face for our clinical operations team, but it was critical to help our organization move toward a digital transformation,” he explains. “Seeing that almost all of our employees had these difficulties demonstrated the need for change.”
Building a business case for eTMF and CTMS
Lozano used the survey results as the basis for his business case for the senior leadership team to invest in eTMF and CTMS. First, he laid out the challenges. Then, he identified the implications and the projected benefits of moving to a unified digital system. “Ultimately, we showed that having a CTMS and eTMF would help us reduce manual tasks, decrease compliance risks, and improve our data and trial delivery to scale up,” says Lozano.
When looking for an eTMF and CTMS, Lozano wanted a single source of truth for clinical operations. “With other potential vendors, they might have had an eTMF or a CTMS, but we could see that the two systems were not fully connected. We would have to build an integration to transfer information between them. We did not have the resources to do that – we needed something that was ready out of the box,” Lozano explains.
Lozano was also looking to improve collaboration between business areas across Radiometer. “Although we initially implemented eTMF and CTMS, we were also looking toward the future. We wanted the potential to have a connected application portfolio across medical affairs, site engagement, and data management. Most vendors do not have that breadth of solutions,” he says. These requirements ultimately led him to recommend Veeva eTMF and CTMS as the preferred solution.
“Although we initially implemented eTMF and CTMS, we were also looking toward the future. We wanted the potential to have a connected application portfolio across medical affairs, site engagement, and data management. Most vendors do not have that breadth of solutions.” –Kevin Valle Lozano, Sr. Clinical Project Manager, Radiometer
Next steps for Radiometer
Now that Radiometer has gone live, the team is planning to release periodic user satisfaction surveys to assess user adoption of Veeva eTMF and CTMS. “Depending on the survey results, we can add configurations to our systems or update processes to address any pain points,” says Lozano. He emphasized the importance of working with Veeva Business Consulting during implementation to address the initial results of the user satisfaction survey. “They have provided end-user toolkits, helped define which of our processes need to be adapted, and helped us navigate new workflows.”
Learn more about how Radiometer utilized user surveys to build a business case.