Veeva Site Connect
Information Sharing
Made Easy
Simplify document exchange for better collaboration and faster trials.
Announced 2020
Status EARLY
Customers 11-50
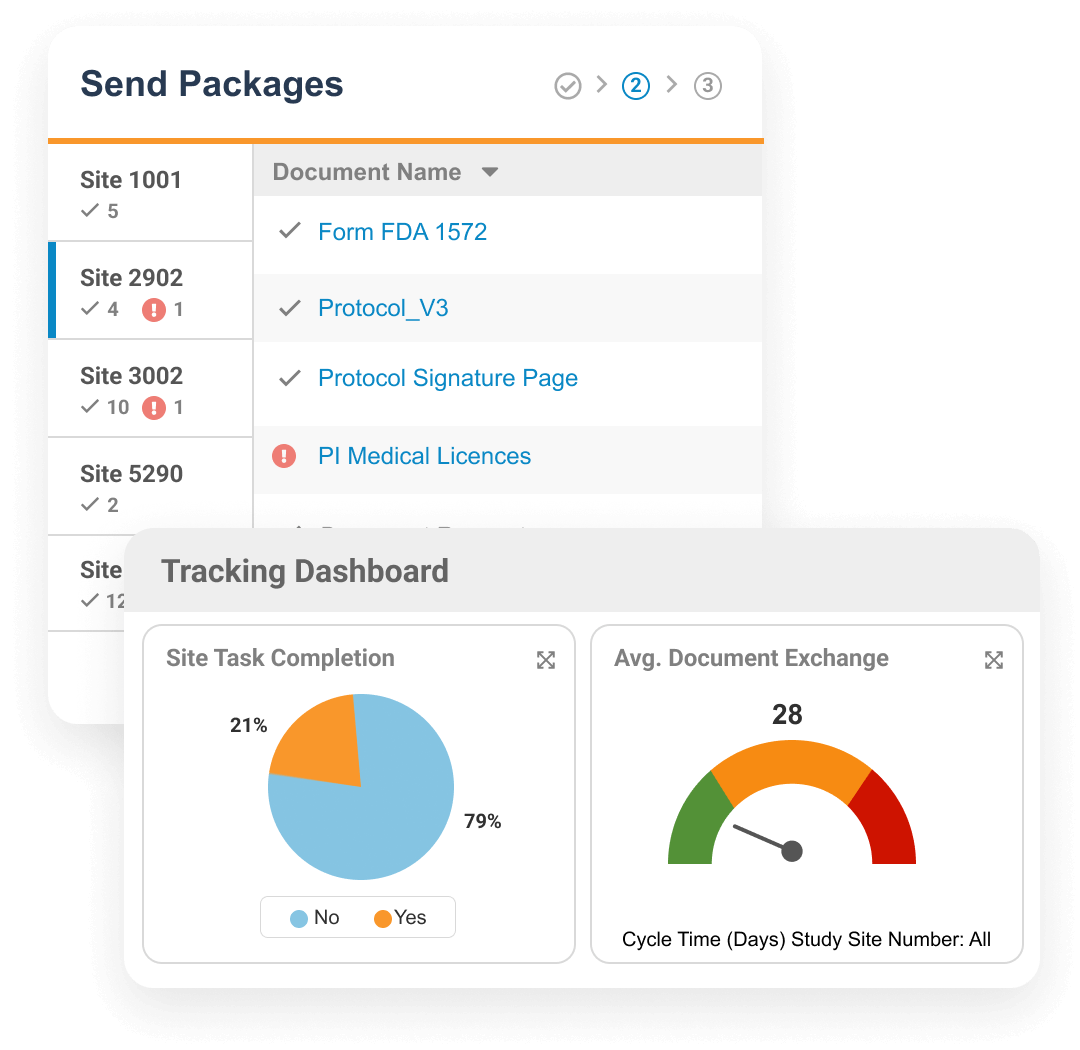
Veeva Site Connect allows sponsors and research sites to collaborate on a trial by automating the flow of information to and from sites during startup, execution, and closeout.
Information flow includes protocols, essential document packages, safety reports, and payment letters. Required media is sent on closeout, including completed CRFs. Information sent and received is automatically filed in eTMF.
Research sites manage tasks and documents using Veeva SiteVault, a free application for sites that allows for efficient cross-sponsor collaboration.
Why Veeva Site Connect
Better collaboration and faster trials
Run faster trials
Automate the flow of trial information to and from sites during study execution to speed trials.Guarantee safety letter distribution
Easily distribute and track safety letters so that principal investigators stay informed.Pay sites faster
Deliver payment letters and automate payment tracking for a simpler, more efficient process.Improve study quality
Easily exchange trial information with sites to reduce manual processes and stay inspection-ready.






