Veeva MedTech Clinical Trials Report Signals Significant Opportunity to Improve Data Delivery and Quality
83% use email, portals, and paper to exchange information with study partners,
slowing study execution and increasing risk of non-compliance
PLEASANTON, CA — Apr. 19, 2023 — Veeva Systems (NYSE: VEEV) today released its first-ever report examining global trends in medtech clinical trials. According to the 2023 Veeva MedTech Clinical Benchmark Report, on-time data entry and data quality are the top challenges for medtech when working with clinical research sites. This can delay trials and increase compliance risk, making improved collaboration with study sites a critical priority for faster delivery of high-quality data.
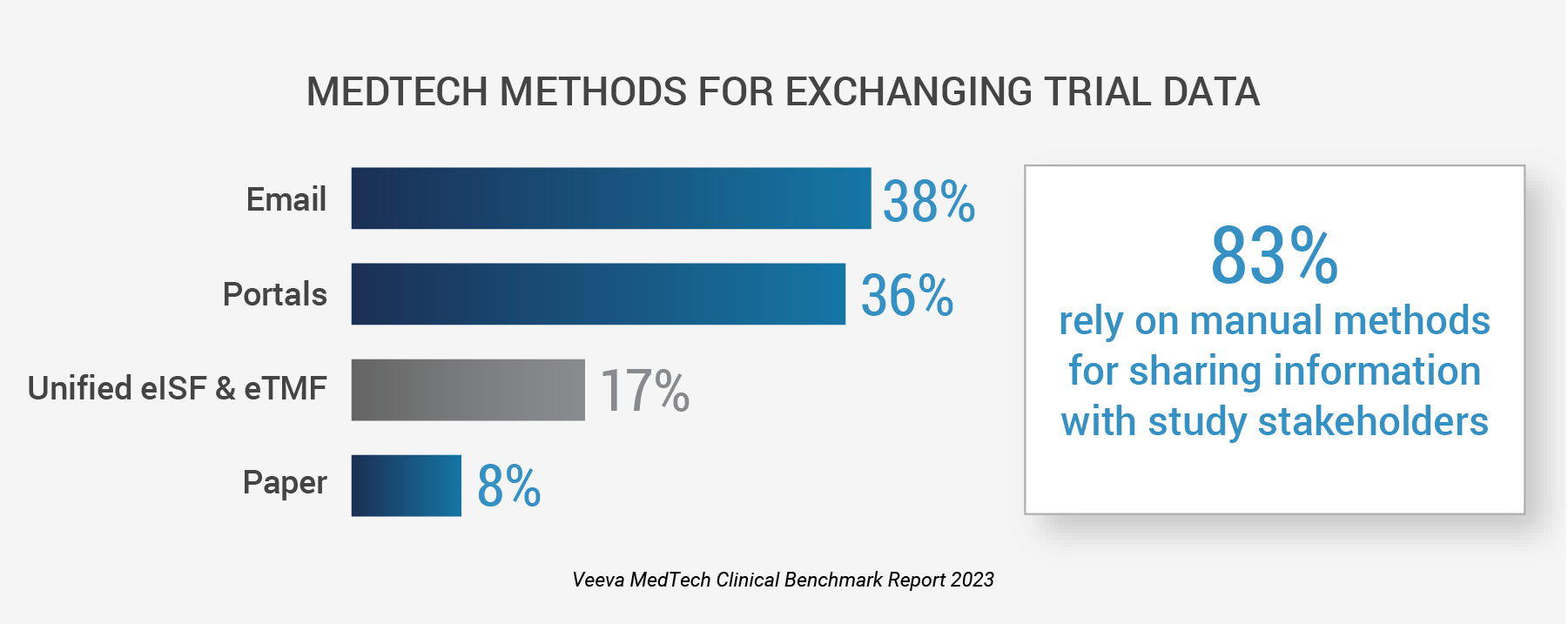
- Disconnected systems remain a crucial issue: More than half of respondents (61%) experience challenges with fragmented clinical systems because of cross-system integration, data management, reporting, and usability. Managing studies on siloed systems can lead to manual errors, duplicate data, and missing files.
- A clear strategy is needed for post-market clinical follow-up (PMCF):: There was no single method for PMCF used by most respondents, with top approaches reported as real-world evidence (21%), literature search (20%), and comparison studies (20%). Without a standard method to meet PMCF, organizations can benefit from developing an end-to-end process that spans clinical, medical, regulatory, quality, and marketing for continuous data gathering throughout the product lifecycle.
- Shift to digital clinical systems accelerating this year: : Over the next 12 months, 45% say shifting to digital clinical systems is a top priority. Establishing a digital and connected technology foundation will make it easier for study stakeholders to work together, increasing trial efficiency, accelerating data delivery, and improving the experience for sites.
“The medtech industry has a significant opportunity to modernize clinical systems and processes for faster access to trial data,” said Kevin Liang, vice president, Vault Clinical strategy, Veeva MedTech. “As more organizations prioritize digital clinical technologies, medtech can improve collaboration with stakeholders and drive trial efficiency, productivity, and compliance.”
The Veeva MedTech Clinical Benchmark study examined how organizations—ranging from emerging to large device and diagnostics companies—manage clinical processes, study site collaboration, and trial data to ensure compliance and speed. This report includes insights from more than 135 clinical medtech professionals worldwide, outlining current challenges and near-term priorities associated with clinical trial conduct See the full annual study which explores how medtech companies manage global compliance and visibility, speed to market, post-market compliance, and modernization.
About Veeva Systems
Veeva (NYSE: VEEV) is the global leader in cloud software for the life sciences industry. Committed to innovation, product excellence, and customer success, Veeva serves more than 1,000 customers, ranging from the world’s largest pharmaceutical companies to emerging biotechs. As a Public Benefit Corporation, Veeva is committed to balancing the interests of all stakeholders, including customers, employees, shareholders, and the industries it serves. For more information, visit veeva.com.
###
Contact:
Deivis Mercado
Veeva Systems
925-226-8821
deivis.mercado@veeva.com