
Alcon enables sites to enter data at a median rate of 1.8 days and over 90% of data entered in less than 5 days
View Customer Story

BD discusses how moving from an eTMF only system to Veeva Clinical has reduced the administrative burden on study teams
View Customer Story

B. Braun shares how clear SOPs and a governance model led to a successful go-live with Veeva eTMF, CTMS, and Payments
View Customer Story
Improve sponsor-CRO collaboration and speed trials
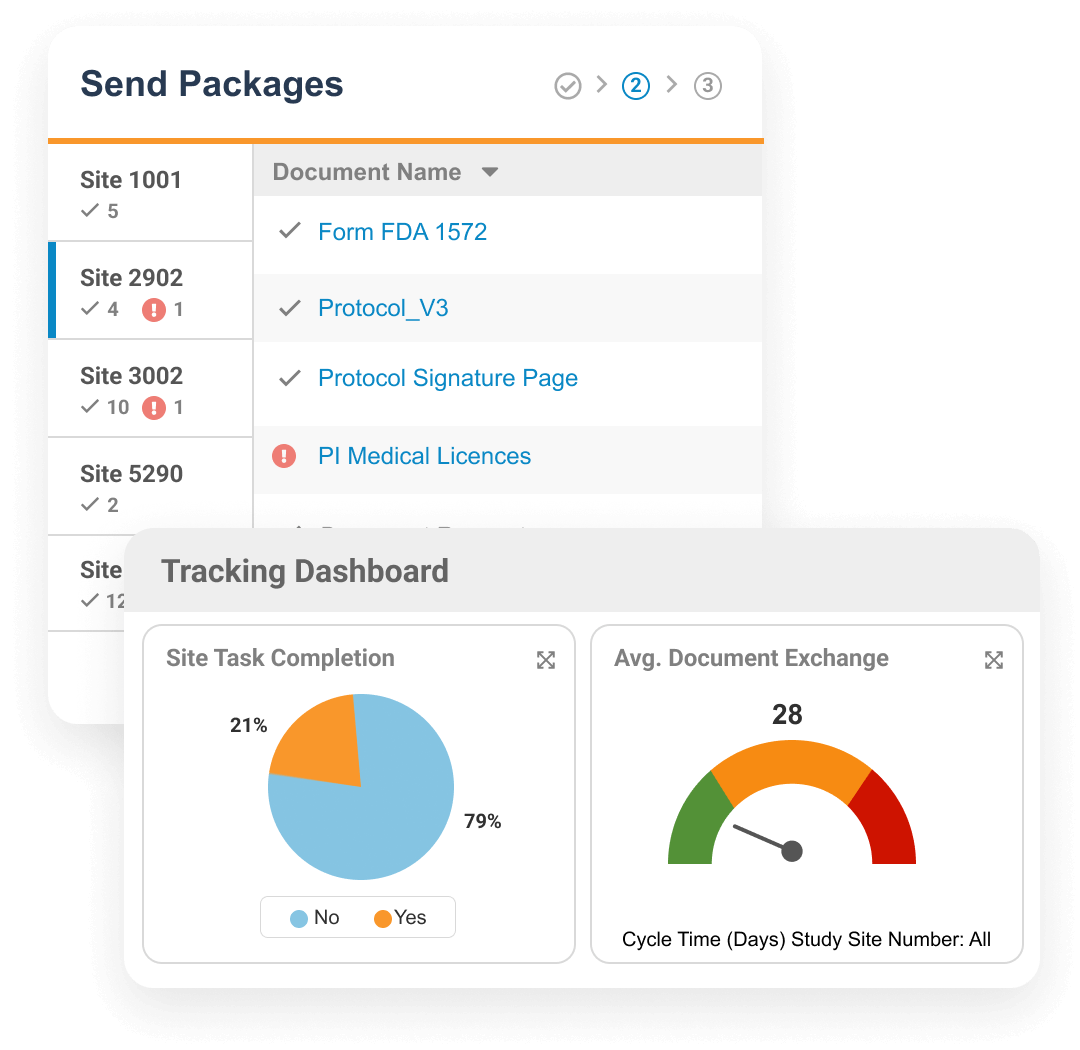
Veeva Site Connect allows sponsors and research sites to collaborate on a trial by automating the flow of information to and from sites during startup, execution, and closeout.
Information flow includes protocols, essential document packages, safety reports, and payment letters. Required media is sent on closeout, including completed CRFs. Information sent and received is automatically filed in eTMF.
Research sites can manage tasks and documents within the sponsor's Vault to drive efficient collaboration and document exchange. This improves sponsor oversight and compliance, while reducing manual processes.
70%
reduction in document exchange and eTMF filing time
60%
reduction in site payment reconciliation
95%
reduction in end-of-study media delivery and tracking time
Site Connect Impact
Run faster trials
Automate the flow of trial information to and from sites during study execution to speed trials.Pay sites faster
Deliver payment letters and automate payment tracking for a simpler, more efficient process.Improve study quality
Easily exchange trial information with sites to reduce manual processes and stay inspection-ready.
Customer Success
Medtechs speed trials with Veeva Clinical Operations






























Watch video
Alcon enables sites to enter data at a median rate of 1.8 days and over 90% of data entered in less than 5 days

Watch video
BD discusses how moving from an eTMF only system to Veeva Clinical has reduced the administrative burden on study teams

Read article
B. Braun shares how clear SOPs and a governance model led to a successful go-live with Veeva eTMF, CTMS, and Payments

View infographic
The MedTech Clinical Benchmark examines how companies are increasing trial activity to maintain product marketability

Watch video
Smith+Nephew shares how a clinical platform drives transparency, study file integrity, and speeds study timelines

Read article
Terumo partners with Veeva Business Consulting to execute their 3-phase organizational change management strategy




