Veeva CDB
Aggregate, Clean, and Transform Clinical Data Across Sources
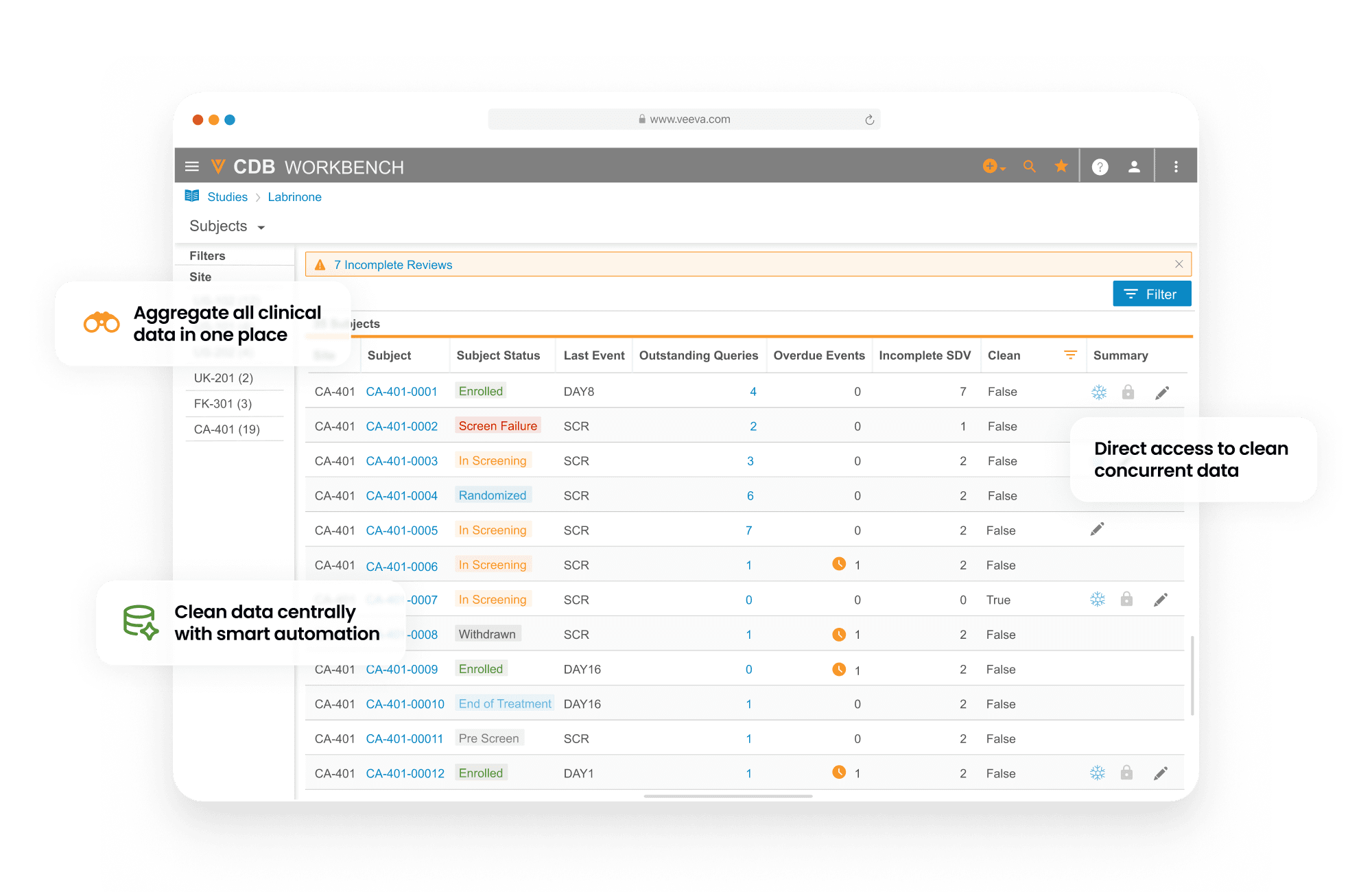
Veeva CDB aggregates, cleans, and transforms clinical data from multiple sources, including third-party EDCs, RTSM, eCOA, labs, imaging, and more. Incoming data is automatically transformed and harmonized to a single package for downstream use.
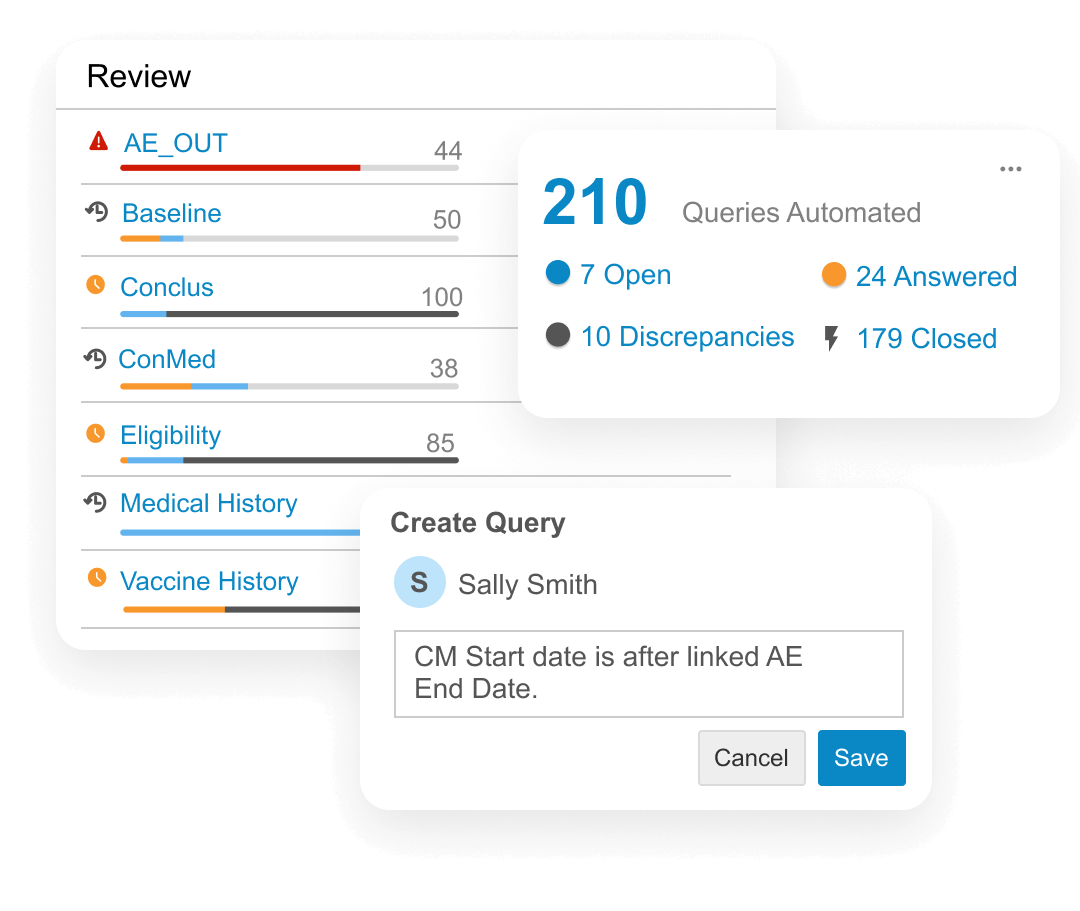
Data managers create and manage queries and communicate with data providers across all sources from a central environment. Data reviewers from all functions, including data management, medical, and analysis teams collaborate in real time. Automatic change detection surfaces new or updated data for review. Automated checks identify discrepancies, create, and close queries.
Oversight teams stay informed of study health using interactive dashboards.
Announced 2018 Status Early Customers11-50
Veeva Clinical Database Aggregates and Cleans Study Data up to 50% Faster
Overview
Aggregate, Clean, and Transform Clinical Data Across Sources
Veeva CDB aggregates, cleans, and transforms clinical data from multiple sources, including third-party EDCs, RTSM, eCOA, labs, imaging, and more. Incoming data is automatically transformed and harmonized to a single package for downstream use.
Data managers create and manage queries and communicate with data providers across all sources from a central environment. Data reviewers from all functions, including data management, medical, and analysis teams collaborate in real time. Automatic change detection surfaces new or updated data for review. Automated checks identify discrepancies, create, and close queries.
Oversight teams stay informed of study health using interactive dashboards.
Impact
Exceed the likely outcome
30%
cleaning effort saved through automation
50%
easier to generate listings
78%
check queries automatically closed
Why Veeva CDB
Access complete and concurrent clinical data
Customer Success
Cuts time to aggregate
and clean study data by 30-50%










Resources
Explore and Learn
Read Features Brief
Veeva Clinical Database Features Brief

Read eBook
Clinical Data Workbenches: A Buyer’s Guide

Read Press Release
Veeva Clinical Database Crosses 200 Study Milestone

Watch Demo
Veeva CDB Product Demo
Read Blog
Beyond the Assembly Line: Mastering the Elements of Clinical Data Science
Read Blog
Clearing the Technology Path to Clinical Data Science

Watch Video
Syneos Health Transforms Clinical Data Management for Their Sponsor

Read Blog
Data Aggregation and the Veeva CDB Ingestion Engine
Read Blog
There is More to CDMS Than EDC

