Veeva Quality Cloud
Modernizing Quality with
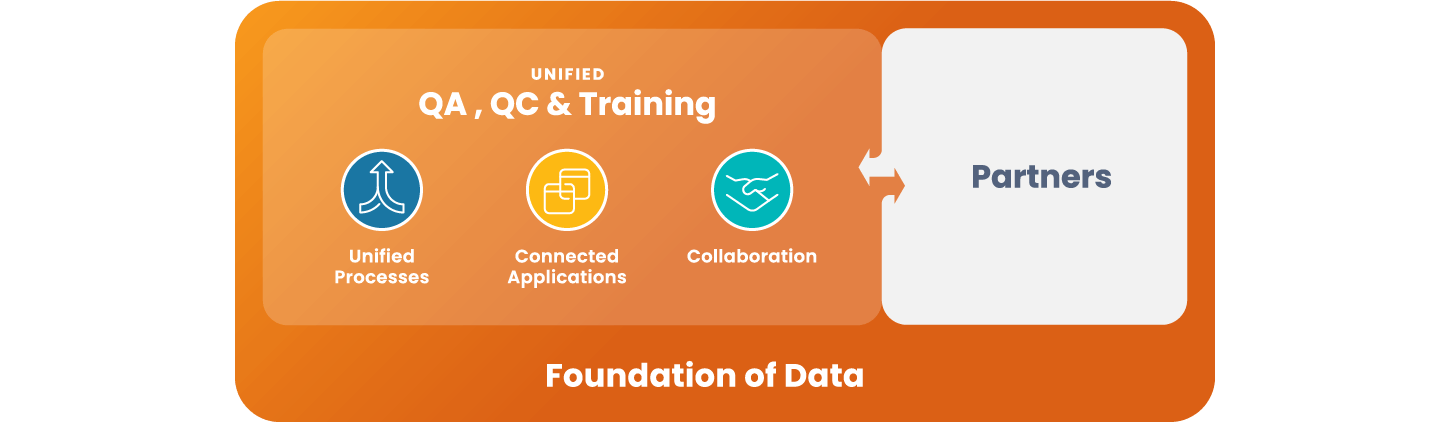
Unified QA, QC, and Training
Remove silos, create efficiency, and drive collaboration.
Watch R&D and Quality Summit, U.S. sessions on Veeva Connect
Optimize Quality. Simplify Processes.
Veeva Quality Cloud accelerates the manufacturing of high quality products to a greater number of patients. The cloud platform unifies applications, processes and partners across content management, training, Quality Management System (QMS) and QC lab solutions (LIMS).
-
Unify Quality
Modernize & create efficiencies
across quality functions -
Connect External Partners
Engage external partners
in standardized processes -
Enable Proactive Quality
Establish a foundation
for advanced analytics and AI
Veeva AI for Quality
Quality Event Agents
Aggregate data across multiple objects to generate narrative summaries for Investigations and CAPA plans.
Document Translation Agent
Generate translations of documents such as SOPs into various languages to improve overall cycle times.
Products
Digitize Quality with Industry Best Practices
Standardize and simplify GxP quality management processes with Veeva Quality.
Processes
Unified QA, QC & Training processes across manufacturing, R&D and partners, minimize quality cycle time with better performance.
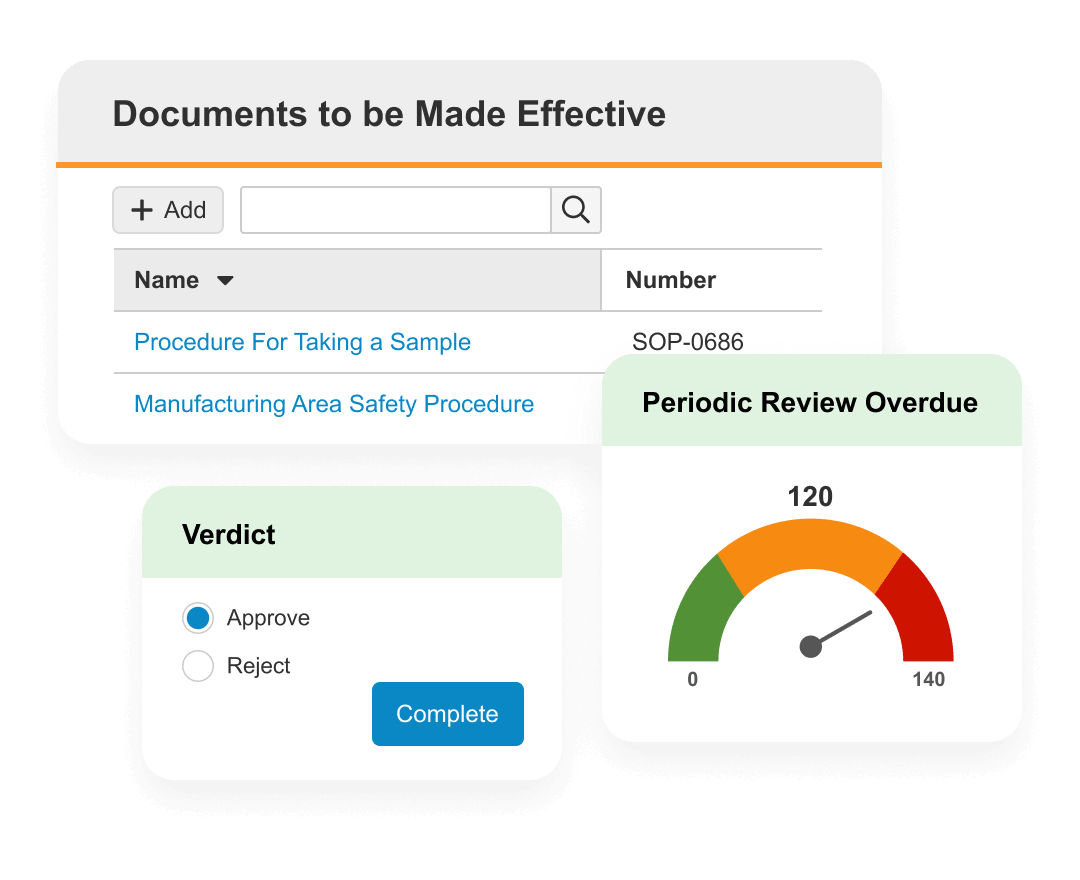
Veeva QMS
Manage quality processes globally for greater visibility and control.
Veeva Product Surveillance
Simplify and standardize postmarket surveillance for medical devices.
Veeva Batch Release
Speed release decisions with confidence.
Veeva Validation Management
Manage and execute paperless validation faster, and at lower cost.
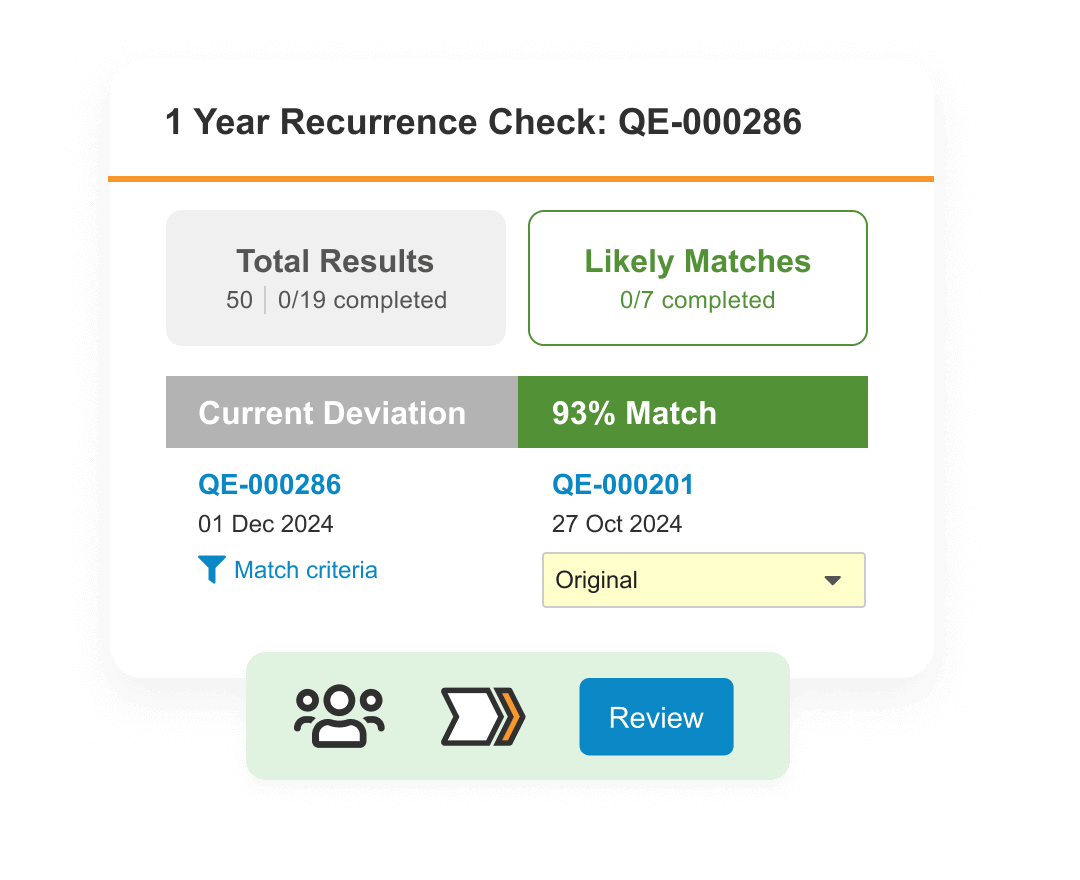
Content
Achieve greater visibility and control over regulated content and data, across partners and all GxPs.
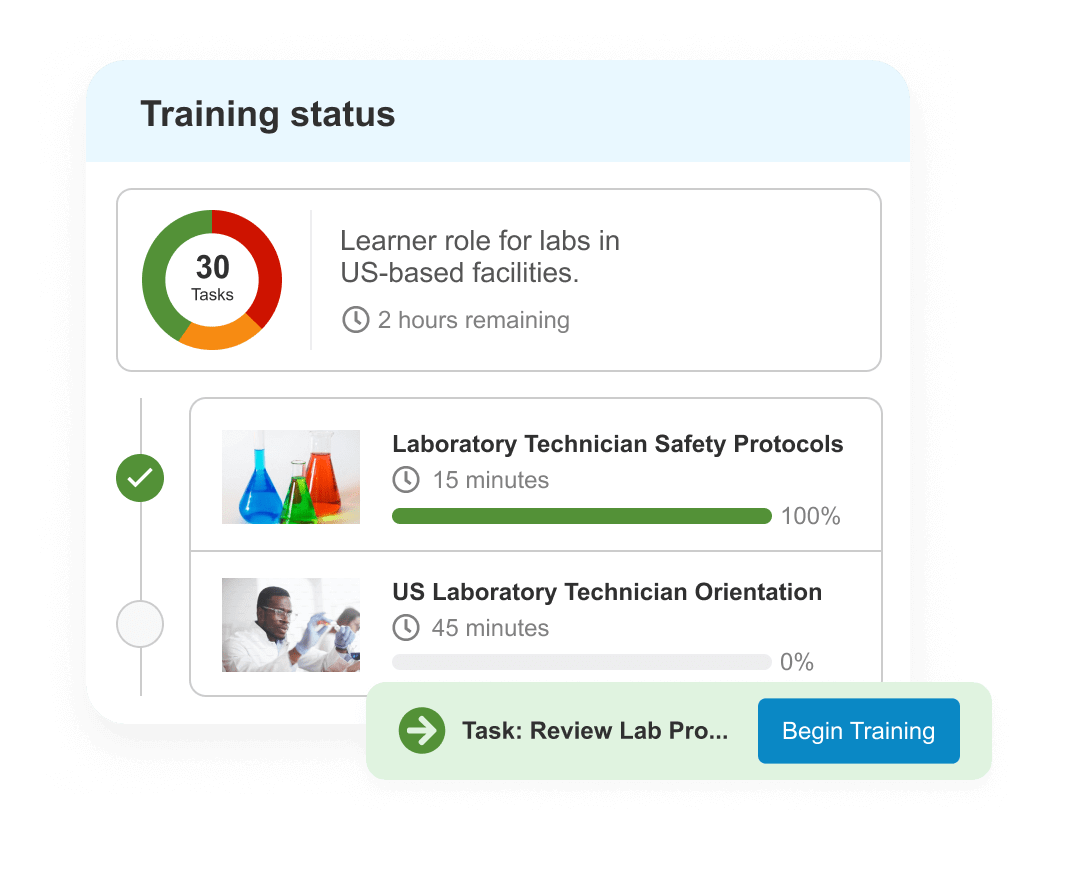
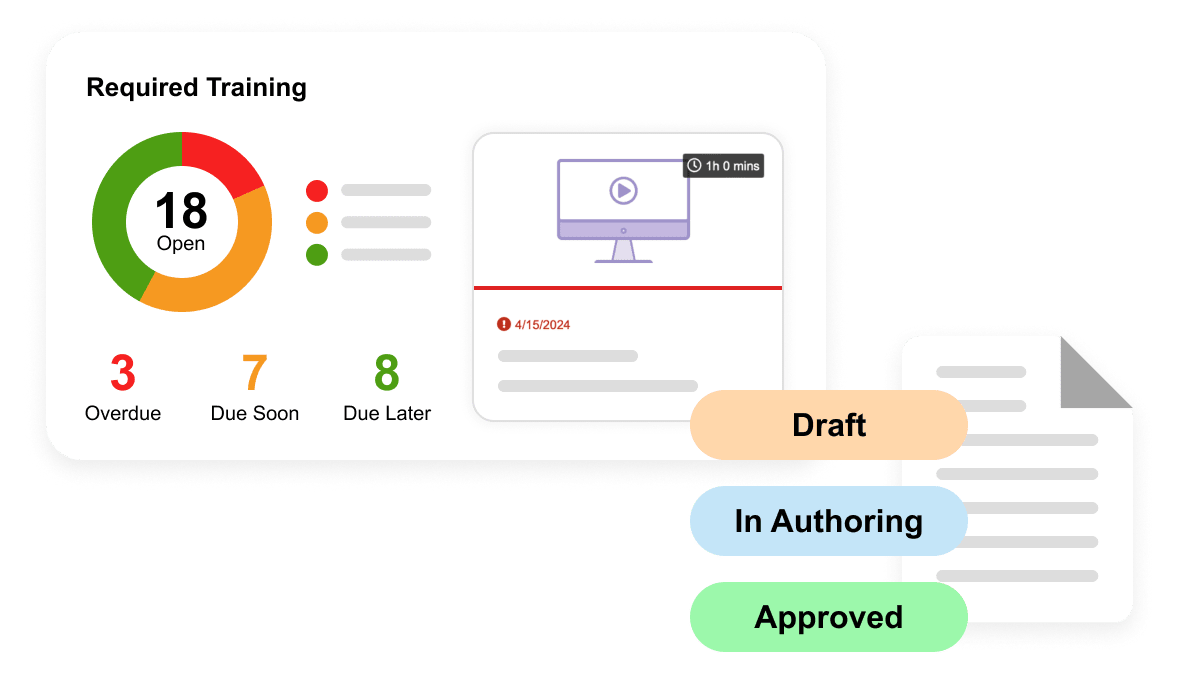
Training
Increase quality training efficiency and compliance with end-to-end training. Automatically verify qualifications, surface content & trigger training as changes occur.
Lab
Modernize Quality Control for greater productivity, improved compliance, and faster batch release.
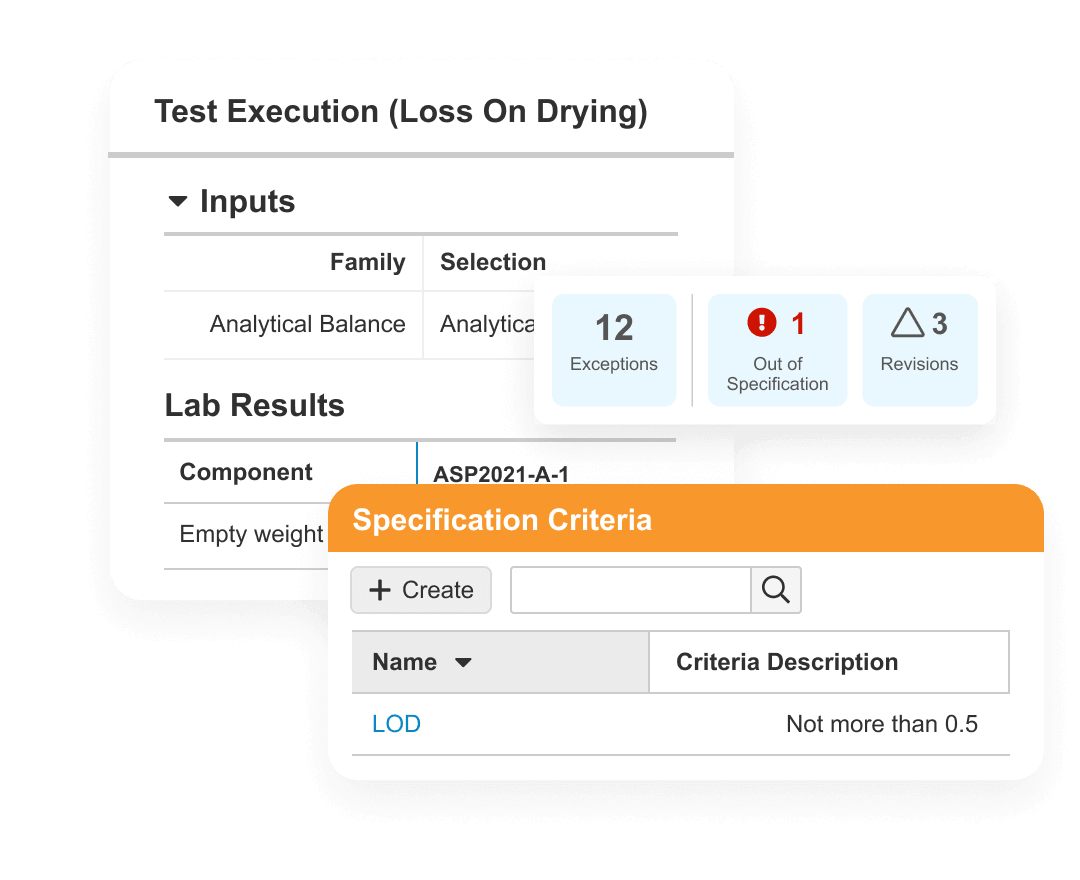
Veeva Quality Basics
For emerging biotechs, Veeva Quality Basics delivers Veeva’s industry-leading quality applications, ready-to-use out-of-the-box with no implementation or maintenance costs.
Veeva Connections
To see a full list of available Veeva Connections, visit the Veeva Development Cloud page.
Veeva Connections are Veeva-delivered integrations for seamless transfer of data and documents between Vaults. Veeva Quality to RIM Connection accelerates the change control process by connecting change control and variation management processes.
Customer Success
More than 600 biopharma, contract manufacturers, generics, and medtech companies
standardize and optimize quality management with Veeva Quality applications.
Are you a Contract Manufacturer or Generics company? Learn More











Resources for Veeva Quality
Learn More
Veeva Quality Cloud Features Brief

Watch Video
Arcellx: Streamlining Content Management and Training with Veeva Quality

Watch Video
medac: Modernizing Quality Drives Business Value and Reduces Cycle Times

Watch Video
Scaling for Growth with a Modern Solution

Watch Video
Haleon: Using AI to Advance Predictive Quality and Risk Management

Read Blog
The Path to Data-Driven Quality Management

Beyond Life Sciences
Veeva QualityOne partners with industries where scale and impact matter most. We help you deliver safe, high quality products that consumers trust—for industries such as Consumer Packaged Goods, Food & Beverage and Chemicals.
To explore quality products for other industries visit the Veeva QualityOne website.

