Veeva R&D Summit: In Highly Regulated Environments Customers are Embracing Agile Methodologies
Agile approaches enable organizations to assess and adapt throughout the development lifecycle. Typically these methodologies are used in software development, and are now being applied to other areas of the business. At this year’s first Veeva R&D Summit, customers heard how organizations are incorporating agile strategies in life sciences to increase flexibility in adapting to new conditions, foster rapid innovation, and swiftly mitigate investment risks.
One hot topic was how to shift from the traditional waterfall model and apply agile methodologies to the R&D process for new products – freeing organizations to innovate and scale quickly in an environment with rigorous procedural requirements. We discussed how to best leverage cloud-based document control to create flexibility in the stage gate process while strengthening compliance. The stage-gate process is a technique used for managing new product development or process improvement. The process is divided into stages or phases, separated by gates, from concept through launch. Taking an agile approach to this model means frequent checks on the acceptance criteria of a product, enabling organizations to fail quickly and rapidly change direction – as many times as necessary, or succeed with confidence.
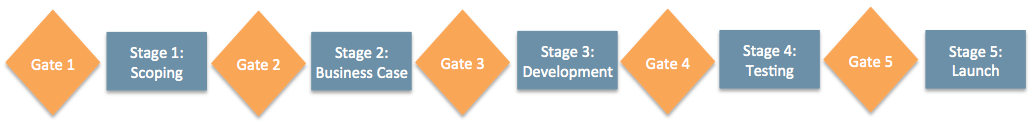
Buying into the agile vision is easy, as many case studies have shown that “too much structure can inhibit creativity”1 and flexibility is a critical success factor. However, making the transition to an agile environment can seem overwhelming. We heard how Zoetis, Natera, and other life sciences companies are successfully adapting to an agile SaaS model, while maintaining a validated and compliant environment. Moving to the cloud has also enabled these customers to quickly take advantage of new capabilities delivered on an ongoing basis, with less effort, as validation tasks for each release are shared between Veeva and the customer.
Harmonizing compliance approaches across three different disciplines: GLP, GCP, and GMP were also explored. Although these regulations are often managed independently, there are many commonalities and sometimes the same data or documents are used across multiple areas, such as work instructions for similar equipment. While the processes differ across these three domains, common data such as product names, materials used, and location should have consistent values, and sharing audit findings and CAPAs ensure similar mistakes are not made. Transparency between these functional groups is even more critical in an outsourced environment where external partners do not have easy access to internal data and documents. Increasing visibility is essential to managing these relationships more effectively and ensuring quality across a complex and distributed product development lifecycle.
I was pleased and excited to see our industry engage in a spirited conversation about moving life sciences forward to better meet the increasingly stringent regulations and challenges we face. As cloud is increasingly leveraged throughout life sciences, there will be more stories on how companies are leveraging the technology in new ways. I can’t wait until next year!
Reference:
1. Ulf Hogman, Hans Johannesson, 2013. Applying stage-gate processes to technology development—Experience from six hardware-oriented companies. Journal of Technology and Engineering Management 30 (2013) 264 – 287.


