Veeva Quality
O Veeva Quality unifica aplicativos de gerenciamento de qualidade, documentos e treinamentos para automatizar e harmonizar processos de qualidade globalmente. Permite que empresas de ciências da vida modernizem o gerenciamento de qualidade e impulsionem inovações operacionais e direciona a conformidade. Todas as partes envolvidas – industria farmacêutica, fabricantes contratados e fornecedores – ganham maior visibilidade e controle acessando uma única fonte autorizada para dados e documentos de qualidade.
Gerenciamento de Qualidade do Início ao Fim
Gerencie processos de qualidade com dados e conteúdos regulados em toda organização e com parceiros.
Maior Colaboração
Facilita suporte a todos os modelos de parceria, alinhando processos globais para automação eficaz.
Total Controle e Visibilidade
Fonte única de informação com total controle e visibilidade dos processos de qualidade, do desenvolvimento à comercialização.
Unificado e Conectado
Uma suíte unificada dentro do Veeva Development Cloud, agilizando processos de negócios multidisciplinares com uma única plataforma na nuvem.
Confira mais detalhes baixando a fixa do produto:Download product sheet
Veeva QMS
Possibilite gerenciamento e visibilidade globais por todo processo de qualidade.
Saiba MaisVeeva Product Surveillance
Simplifica o acompanhamento pós-mercado de dispositivos médicos.
Saiba MaisVeeva QualityDocs
Oferece controle de documentos BPx com revisões e aprovações de fluxo de trabalho automatizadas.
Saiba MaisVeeva Training
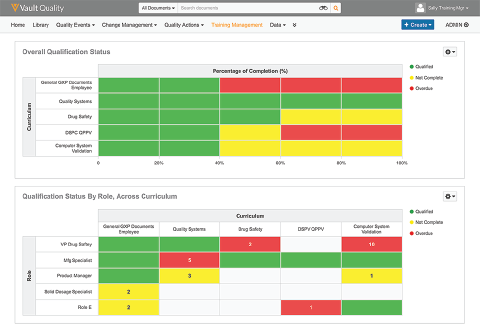
Automatiza o gerenciamento de treinamento para conformidade BPx e qualificações baseadas em funções.
Saiba MaisVeeva Connections

Veeva Connections são integrações fornecidas pela Veeva que transferem perfeitamente dados e documentos entre aplicações Veeva. Para equipes de qualidade, o Veeva Quality to RIM Connection diminui o tempo geral desde a criação do evento do controle de mudanças até a implementação.
Para ver toda a lista de Veeva Connections disponíveis,
visite a pagina do Veeva Development Cloud.
Clientes do Veeva Quality
Mais de 300 empresas de ciências da vida utilizam a solução Veeva Quality para modernizar o gerenciamento de qualidade