Increase efficiency and gain
oversight of pharmacovigilance
agreement obligations
Veeva SafetyDocs enables more efficient
management of pharmacovigilance
agreements (PVAs) and safety data exchange
agreements (SDEAs) and provides oversight of
PVA/SDEA obligations.
With secure access and customizable
workflows, Veeva SafetyDocs makes it easier
to collaborate with external partners, manage
multiple PVAs/SDEAs globally, and track
obligations for improved compliance.
Business Benefits
Improve partner adherence.
Gain oversight of partners
and PVA obligations with task
workflows and customizable
reports and dashboards.
Efficiently monitor activities for
timely completion to reduce
compliance risk.
Efficient PVA lifecycle
management.
Centralize PVA documents and
workflows in a single system
to manage all of your PVAs and
SDEAs from authoring through
termination.
Always use the right version.
Automated version control
ensures the correct PVA
version is always used and
improves inspection readiness.
Features
Automated Obligation Scheduling
Automate recurring PVA activities to support real-
time reporting and reduce manual effort.
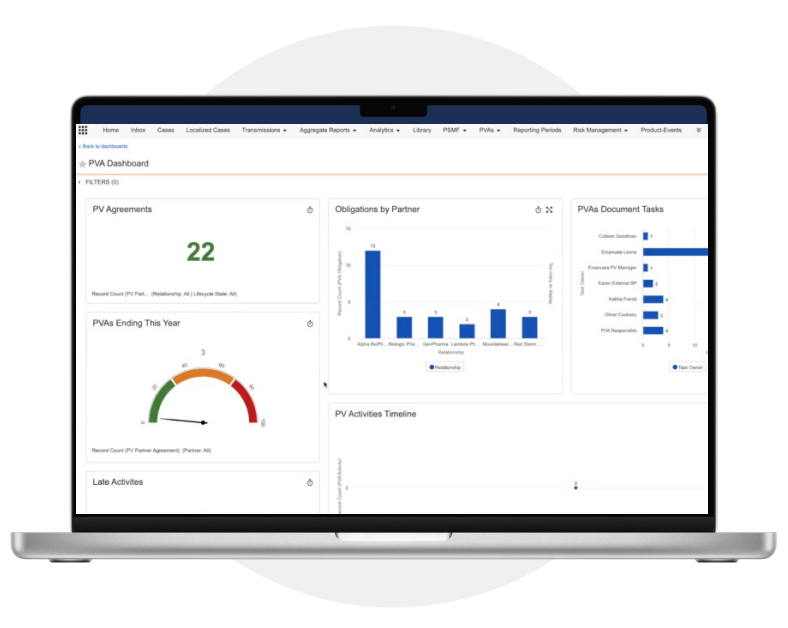
Reports and Dashboards
Track operational metrics to determine the status
of PVA obligations, as well as oversight of partner
activities and compliance.
Real-time Document Distribution
Securely share documents with partners via email
or a Vault workflow and track their activity, including
receipt and acknowledgment.
Configurable Workflows
Automatically generate tasks for external partners
to ensure agreed-upon obligations are completed
timely.
Version Control
Automate versioning and easily compare documents
to previous versions to see how the content was
changed.
User Access Management
Set up secure user access roles to define what
content users are allowed to view or edit and what
activities they can complete.
Automated Notifications
Receive automatic alerts when a pharmacovigilance
agreement is due to expire.
Template Management
Create and store PVA templates to speed up the
time to draft new pharmacovigilance agreements.
Watch demo to see how to improve oversight and tracking of PVAs.
