Clinical Researcher – November 12, 2019 (Volume 33, Issue 9)
Henry Levy, Veeva Systems
Clinical teams are under enormous pressure to
reduce cycle times and accelerate trials to
completion. It costs an estimated $2.6 billion
to get a drug to market1 —twice as much now
compared to 15 years ago. Study delays can
contribute $800,000 to $8 million per day,
highlighting the need to drive more efficient
clinical trials.
At the same time, studies have become
increasingly complex and the number of
endpoints and procedures are growing,
making data management timelines longer.
Average times to build and release databases
and lock study data have both increased over
the last 15 years.2
Three biopharmaceutical and biotechnology
organizations—Vertex Pharmaceuticals Inc.,
Lotus Clinical Research, and Cara
Therapeutics—are reversing this trend. By
adopting a modern electronic data capture
(EDC) system and streamlining clinical data
management processes, the companies have
improved performance throughout the clinical
trial lifecycle.
Vertex Pharmaceuticals:
Shorter Build Times, Higher
Operational Excellence
Operational excellence is a core principle at
Vertex. Since 2017, the global biotechnology
company has been outperforming industry
averages for data entry and data lock cycle
times; its EDC data are typically entered by
sites within 48 hours and data locks are
completed within 15 to 18 days. Only its
database build times, averaging 12 to 14
weeks, were slower than desired.
The company set a goal to shorten build times
to six to eight weeks, with completion to
always come before the “first patient first visit”
milestone. The data management team
needed a modern, agile EDC that would
support process changes to reduce build times,
while maintaining the highest standards.
“You can’t compress a 12 to 14–week timeline
to six weeks by just working faster,” said Vikas
Gulati, executive director for clinical data
management and metrics at Vertex. “Likewise,
there is no benefit in shortening database build
times if it impacts quality or extends lock
times.”
Traditionally, Vertex would author and provide
its EDC vendor with a detailed specification
document, commonly called “a spec,”
detailing study build requirements. To shift
toward spec-less design, its current EDC
vendor built a study template based on
Vertex’s standards library for electronic case
report forms (eCRFs). With the study template,
the vendor now works directly from the study
protocols. This eliminated the process of
authoring and reviewing a spec, saving Vertex
weeks with each study. Adding crossfunctional
design reviews to the build process
preserves the thought, rigor, and oversight
that goes into ensuring the EDC is capturing
the correct data and running the appropriate
checks.
Reusing forms from a library or a template
study greatly increased the efficiency and
lowered the effort of building studies,
especially for an organization like Vertex that
focuses heavily in one therapeutic area. In the
first two studies with its current EDC solution,
only one net new form needed to be created.
The rest were pulled and modified from the
standards library.
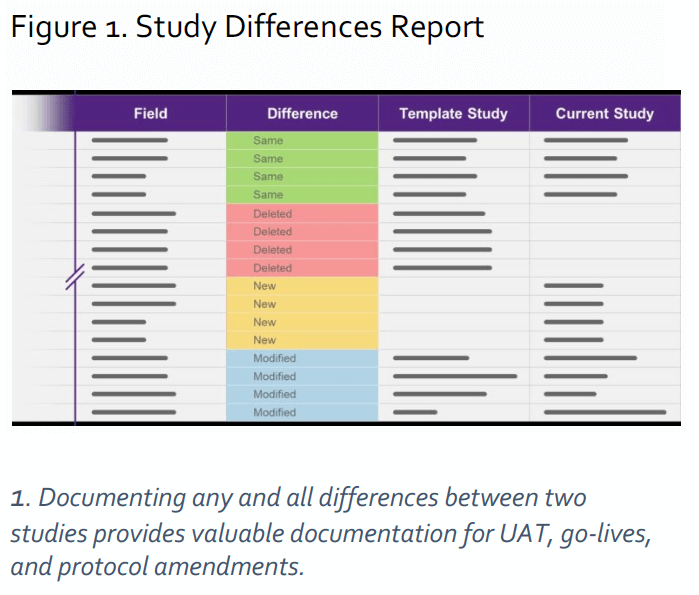
Tied to the template study and the re-use of
forms was the adoption of a risk-based
approach to user acceptance testing (UAT)
enabled by an EDC innovation called a
differences report (see Figure 1). The
differences report allows the data
management team to see differences between
two studies, including additions, omissions, or
changes. Using the template study as
reference, Vertex no longer performs UAT on
the forms that were previously tested. The reuse
of forms from the template study
dramatically reduced the amount of testing
needed.
For the forms that still needed to have UAT
performed, Vertex abandoned the traditional
“ping-pong” approach to UAT—a process of
sending a casebook back and forth between
the vendor and study team that could take up
to two weeks per round. Instead, Vertex
adopted a live, real-time UAT model in which
the stakeholders of the study gather in a single
room with the vendor to make real-time
updates directly to the casebook, saving time
and aligning stakeholders on the changes.
“Live UAT updates are a game-changer,” said
Gulati. “By providing feedback, fixing
problems, and testing updates immediately,
we can eliminate three to four weeks from our
timeline.”
By modernizing its clinical data management
processes, Vertex has reduced average build
time to 7.5 weeks, meeting its goal and
continuing a track record of operational
excellence.
Lotus Clinical Research:
Streamlined Data Quality
Reviews
In addition to being a full-service contract
research organization (CRO) for analgesic
studies, Lotus Clinical Research operates a
state-of-the-art research site. It aims to lead
the pain market for CRO and site services by
introducing and validating technologies that
will improve analgesic study design and
conduct.
The company adopted an EDC solution that
was easier to use for site personnel, data
managers, and clinical research associates
(CRAs) and that improved efficiency across
teams. With a capability in its EDC system that
allows patient listings and forms formerly
created for the use CRAs at a significant cost of
time by data managers to now be
automatically generated within the system
itself, Lotus automates the tracking and
sorting of patient data for entry, source data
verification (SDV), and review.
“I used to send my monitors all the reports
before they went on their monitoring visits,”
said Andrea Krueger, a data manager for
Lotus.
“Now my CRAs told me not to send them
anymore, because [in our EDC now], you click
on a subject casebook and it shows what
requires SDV and what queries need to be
addressed. It’s all in the system already.”
This functionality provides benefits to data
managers and CRAs, saving both monitoring
time and effort. Instead of cross-referencing a
spreadsheet, CRAs work directly within the
EDC. The system filters out the visits and
forms that haven’t been completed or that
were already reviewed, listing only the data
that need SDV and queries that need answers.
Data managers can also save time preparing
for meetings with clients. The reports and
dashboards that show progress and status of
data entry are also fully automated in the
cloud-based EDC system.
“Our clients really like receiving the status
reports,” said Krueger. “They can see the
status of SDV and data reviews, look at the
query reports and status, and who opened and
closed queries. The dashboards have saved me
an enormous amount of time as well. Before, I
would pull reports, calculate the metrics, and
plug those into our template. Now, I’ve
configured my dashboard to show that exact
same data automatically. We simply pull up
our dashboard during the sponsor calls and
walk them through the status, and we can
double click to drill down into any of the
metrics if they have questions.”
A user-friendly interface also makes data entry
easy for clinical research coordinators.
Automated task lists and interactive taskscrolling
allows the site staff to complete their
work more efficiently and with fewer errors.
“Our [system] gives site personnel a userfriendly
interface that takes them directly to
what’s needed so they no longer have to click
through casebooks and find where they left
off,” said Jennifer Nezzer, director of
biometrics at Lotus Clinical Research.
Cara Therapeutics:
Trial Data On Demand
As a small clinical-stage biopharmaceutical
organization, Cara Therapeutics outsources its
studies to CROs, often using different vendors
for different studies. It’s a growing trend in
response to the rising complexity and cost of
running a trial. It is estimated that by 2020,
nearly three-quarters of trials will be
outsourced to CROs.3
Outsourcing trials created delays in accessing
data because the company was dependent on
CROs to handle periodic exports of the data.
Working with multiple CROs also introduced
variability into the CRFs and datasets, creating
more work downstream for the programming
team because each CRO used its own EDC and
standards.
Cara wanted the benefit of using CROs that
specialized in specific clinical areas, while also
maintaining control over CRFs. By providing
CROs with a cloud-based EDC, Cara was able
to standardize and align data collection from
multiple CROs.
“Our new clinical data management system
gives us control over our casebooks and
consistency in our data when working with
different CROs,” said Evelyn Dorsey, associate
director of data management for Cara
Therapeutics. “We’ve used the system with
more than five different CROs, and they have
all been impressed with the speed of building
studies and making mid-study changes.”
Rather than waiting until the end of the month
for data transfers from a CRO, now Cara can
analyze and investigate data continuously by
simply signing into its EDC. Cara has constant,
direct access to its trial data in real time. With
higher visibility into study status, it also sees
the operational reports showing the status of
data collection and cleaning without having to
ask the CRO for a separate report.
Flexible reporting within a cloud-based EDC is
also valuable when working with multiple
CROs. Sponsors see operational data in a
consistent way across studies, while each CRO
can see the study metrics and reports
according to its own preferences.
“We have six or seven different CROs working
with us, and they have three or four team
members within the data management group
working on each of our studies,” said Dorsey.
“Flexible reporting is really valuable to us, as
we are able to share our custom reports from
other studies as a baseline. Each team member
with access to the EDC can customize their
view of that report however they like
it, without the need request such reports from
the programming team, which also means cost
savings for Cara.”
EDC Innovations Advance
Clinical Trial Efficiency
There are dramatic changes under way in
clinical data management, and yet the
challenges associated with EDCs and
managing site data have persisted for years.
The recent advancement in EDC innovations
are helping companies like Vertex, Lotus, and
Cara solve persistent challenges with lengthy
build times, inefficient SDV, and disconnects
between sponsors and their CRO partners.
Advancements in clinical data management
will continue to help CROs, sites, and sponsors
toward modernizing trial processes and
reducing the speed and cost at which we can
bring treatments to patients.
About the Author
Henry Levy (henry.levy@veeva.com) is
general manager for Veeva Vault CDMS with
Veeva Systems, president of the industry
standards groups Align Biopharma and Align
Clinical CRO, and a board member of the
Alliance for Clinical Research Excellence and
Safety.
References
1 DiMasi JA, Grabowski HG, Hansen RW.
2016. Innovation in the pharmaceutical
industry: new estimates of R&D
costs. J Hlth Econ 47:20–
33. https://dukespace.lib.duke.edu/dspace/bitstream/handle/10161/12742/DiMasi-Grabowski-Hansen-RnD-JHE-2016.pdf?sequence=1&isAllowed=y
2 Young R. Data management in the
face of growing trial
complexity. Contract
Pharma. https://www.veeva.com/wp-content/uploads/2019/05/Article_-Vertex-Leverages-Innovative-Processes-to-Speed-Data-Management.pdf
2 Research and Markets. 2018. Global
market for contract research
organization (CRO)
services. https://www.researchandmarkets.com/reports/4704012/globalmarket-for-contract-researchorganization#
rela0-3081044
