Efficient, Inspection-Ready TMF Management
Veeva eTMF provides real-time inspection readiness, full visibility into TMF status, and access for all study partners. Sponsors get the clarity they need to oversee trials more effectively. CROs gain the flexibility
and control required to operationalize their SOPs and efficiently populate the eTMF. Auditors get easy online access with a dedicated role.
Veeva eTMF promotes the highest levels of TMF quality, access, visibility, and control.
Business Benefits
-
Real-time inspection readiness
Business-specific workflows ensure TMF content gets managed in real-time, enabling accurate reporting and better decision-making. Organizations can feel confident that their TMFs are complete at all times, eliminating the need for rework at the end of a study. -
Streamline collaboration
Give study teams a real-time view of TMF completeness to help sponsors, CROs, and sites work together to
accelerate trials. -
Unify clinical operations
Speed trial execution by leveraging the most comprehensive group of clinical applications on a single cloud platform.
Active TMF
Active TMF management ensures all TMF documents, related information, and processes are managed in the same
system, in real-time, as they are being executed. As a result, TMF management is automated and in a constant state of inspection readiness.
The entire document lifecycle can be tracked, providing access to a greater set of metrics and data to inform business decisions. Challenges and bottlenecks can be corrected during the course of the study, and manual rework at the end is eliminated.
With an active TMF, adherence to study SOPs and regulatory requirements are not afterthoughts but instead part of an ongoing process to ensure your TMF is always inspection-ready. Veeva eTMF is the only electronic trial master file that enables an active TMF operating model.
-
TMF Reference Model support
Veeva eTMF supports the TMF Reference Model for the documents, properties, relationships, and hierarchies for both core and recommended documents. -
Risk-based document QC
Streamline quality checks by assigning risk levels to document types and setting sampling percentages for each level. The system automatically assesses documents to determine if a QC is needed, focusing on the most critical content while supporting traceability for the audit trail. -
TMF Transfer
Enable study teams to quickly and accurately transfer TMF documents without manual end-of-study migrations.
With a single click, completed study country, study site, associated TMF documents, and audit trail information are seamlessly transferred between sponsor and CRO. -
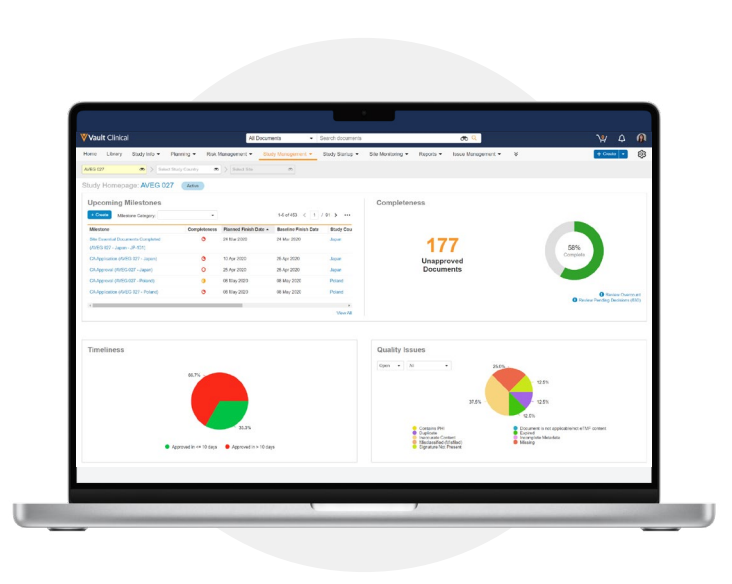
TMF Homepage
Get a high-level overview of your TMF milestones and completeness with the TMF Homepage. Use real-time
dashboards and reports to answer questions about progress and completeness, make proactive decisions, and remedy process bottlenecks. -
Completeness reporting
Identify the documents that need to be collected for a given study with the Expected Document List (EDL). This
functionality empowers users to identify missing documents and take action to upload files directly via drag and drop. -
Real-time collaborative authoring
Support real-time collaborative authoring on all clinical documents in a compliant way with a seamless integration between Veeva and Microsoft Office Online. -
TMF Viewer
Search and access all document versions, as well as export data to Microsoft Excel, with the TMF Viewer. It
provides a real-time view of documents during a study and a full historical picture after study completion. -
Document quality check workflow
Initiate quality checks at the appropriate time to improve accuracy on an ongoing
basis. Users can review document content and metadata simultaneously for each document, making the process
easier and more efficient. -
Global Health Authority submission support
Veeva eTMF automatically creates submission-ready files and captures details relevant for submissions
processing. This eliminates significant downstream processing and removes unnecessary time and expense. -
Timeliness tracking
Calculate the amount of time it takes between two different actions, such as document creation date and document approval date. Timeliness metrics show how quickly departments and partners get documents into your TMF and enable you to review and act on areas of concern. -
Connectivity with LMS
Simplify and automate training and reconciliation with the seamless connection between Veeva eTMF and Veeva Study Training. -
TMF Archive
Accelerate TMF archival with a singleclick archival initiation process. Plus, leverage built-in prerequisites to ensure archival readiness, control access to archived data and documents, and search, filter, and report on archived content.
About Veeva Clinical Operations
Veeva Clinical Operations empowers clinical teams with a unified platform for efficient trial execution.
Streamlined processes and improved data visibility from startup through closeout accelerates timelines
and enhances collaboration across sponsors, sites, and CROs.
