Veeva Vault eSource
Looking for clinical operations solutions?
Click here
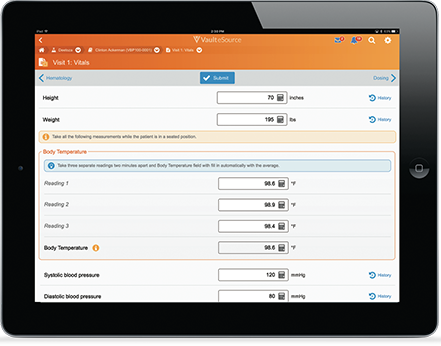
Veeva Vault eSource enables clinical sites to enter patient data directly into an easy-to-use mobile application to eliminate transcription – delivering immediate data quality and reducing wasted time and cost. Veeva is the only provider that delivers eSource and EDC on the same platform, enabling real-time collaboration between sponsors, CROs, and sites on source data.
Vault eSource joins Vault EDC, Vault CTMS, Vault eTMF, Vault Study Startup and Vault SiteExchange as part of the Vault Clinical Suite, the only suite of unified cloud applications for clinical operations and data management—spanning study start-up to archive.
Vault eSource is planned for availability in 2019.
BENEFITS
- Get quality data right from the source: Empower users to make confident data-driven decisions with faster insights, improved data quality, and real-time access. Investigators spend less time on administrative tasks and more time seeing patients.
- Reduce trial costs: Eliminate transcription of data from source documents to EDC, significantly reducing source data verification by clinical monitors.
- Accelerate trial time: Speed clinical trial cycle time by reducing on-site monitoring, gaining immediate access to source data, and decreasing data verification.
- Unify clinical: Leverage the most comprehensive suite of clinical applications on a single cloud platform to unify clinical operations and data management.