Veeva MedTech Suite
Veeva MedTech Suite improves speed and agility throughout the device and diagnostic lifecycle.
Medical device and diagnostic companies are struggling to keep up with changing regulations and the growing demand for clinical data is draining resources. Vault Medical Device Suite streamlines the device and diagnostic lifecycle for greater efficiency and compliance.
BENEFITS
Faster time to market- Improve efficiency and visibility by driving end-to-end processes from development through commercialization
- Reduce time to market for new devices and diagnostics
- Gain real-time data visibility that goes beyond audit readiness
- Stay current with the latest regulatory requirements with easy and automatic upgrades
- Maintain visibility and control over critical compliance processes for audits and inspections
- Demonstrate product value for a competitive advantage
- Disseminate clinical research findings quickly and clearly

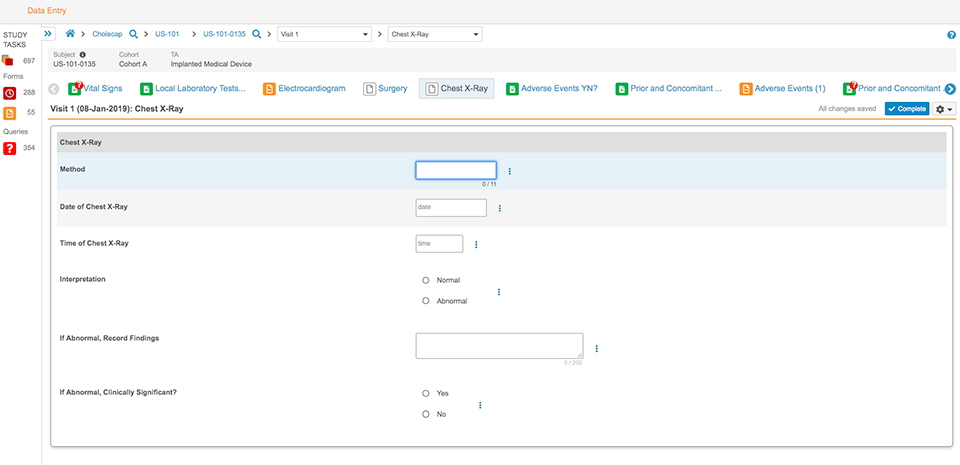
Clinical Data Management
Vault EDC accelerates study cycle times with faster builds, easy amendments, intuitive data capture, and next generation monitoring and data review.
Clinical Operations
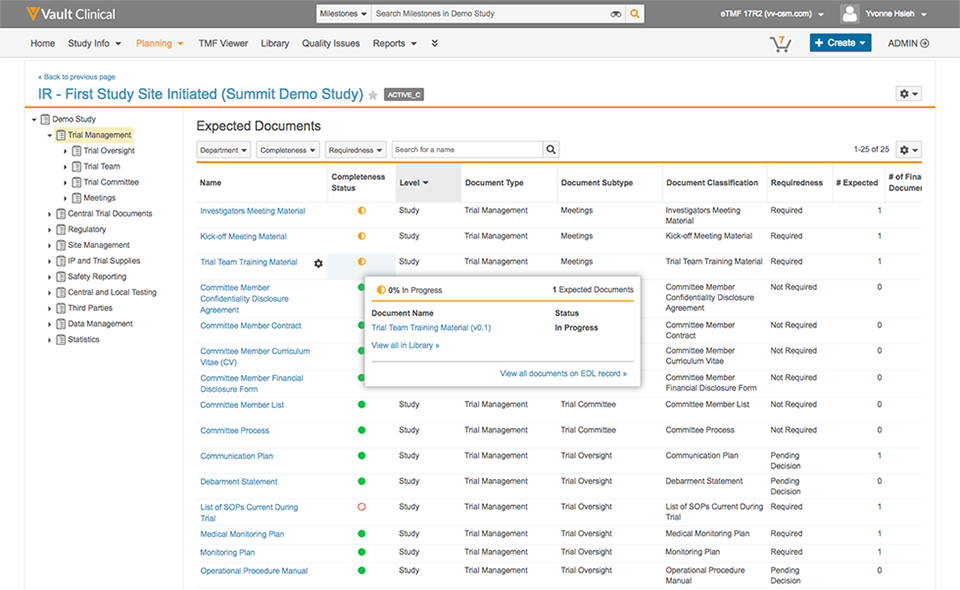
Veeva Vault eTMF enables active TMF management for real-time inspection readiness, visibility, and control.
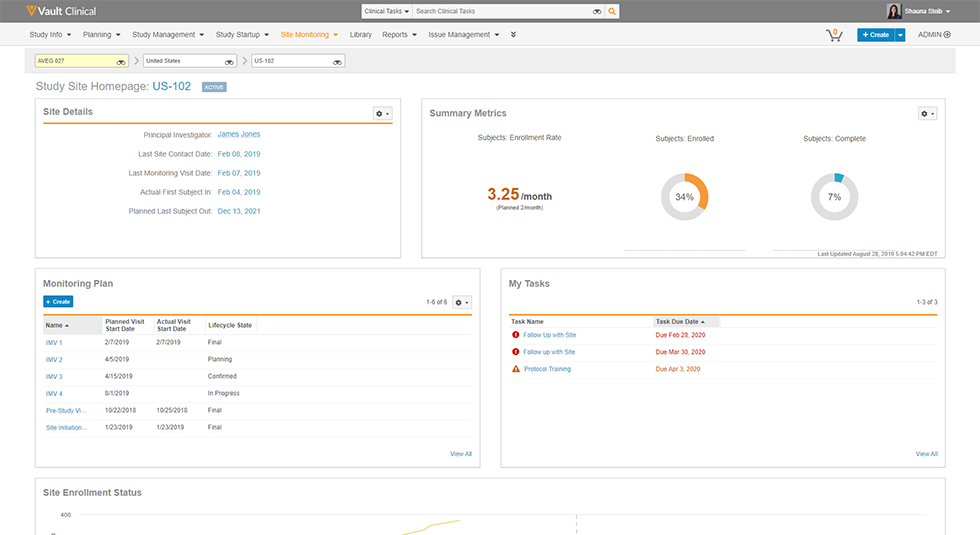
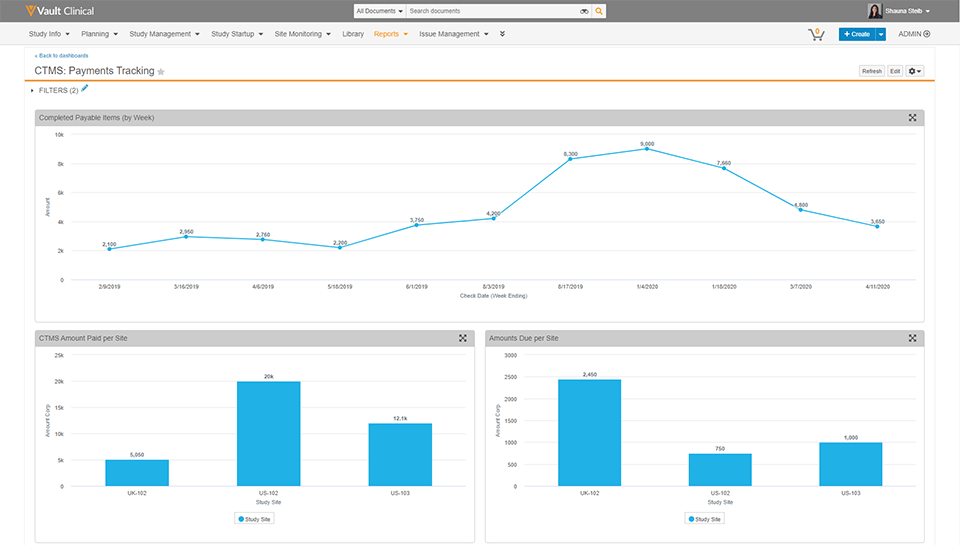
Vault CTMS unifies clinical information, documentation, and processes globally to reduce complexity, increase transparency, and speed time to critical decision making.
Vault Payments speeds payments to clinical research sites and provides financial visibility to all study partners.
Quality
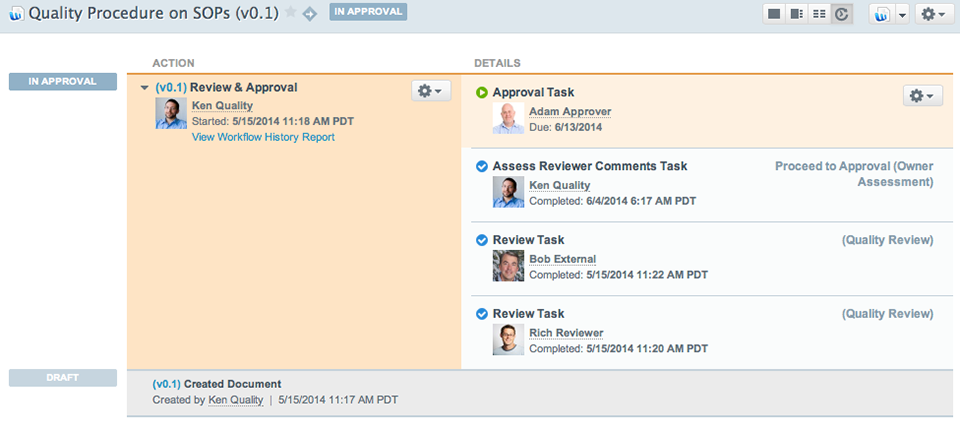
Vault QualityDocs provides a single global source for all quality, manufacturing, validation, and other GxP documents. Vault’s state-of-the-art technology and UI improves adherence to—and reduces the burden of—GxP compliance.
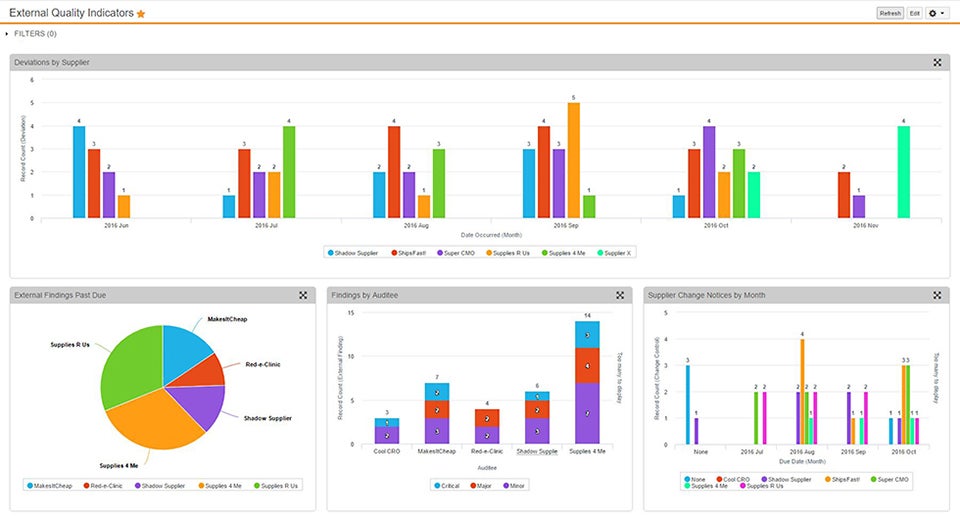
Vault QMS provides global management of quality processes – for all parties – enabling end-to-end control and visibility. Easily support proactive management initiatives and Deviation, Internal/External Audit, Complaint, Lab Investigation, Change Control, and CAPA processes, or configure your own.
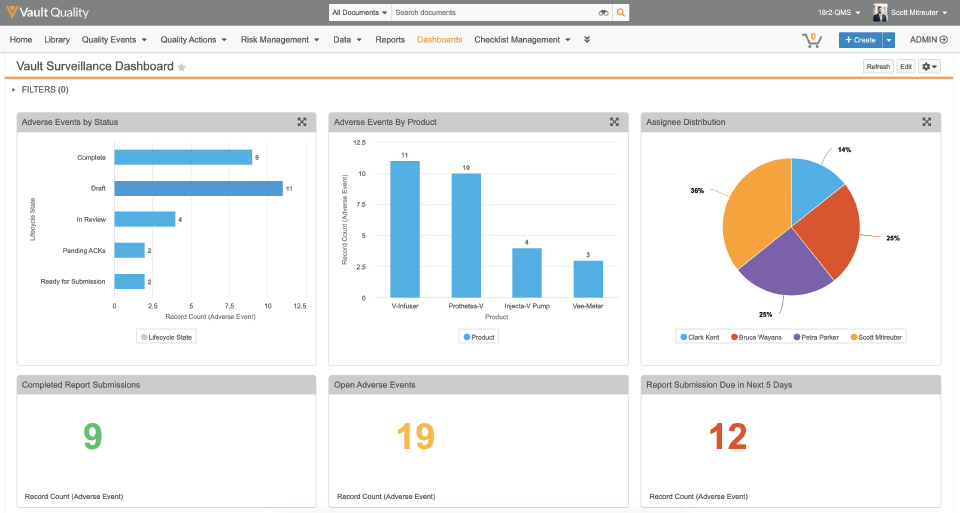
Vault Product Surveillance simplifies and standardizes postmarket surveillance for medical devices, improving product safety, reliability, and quality. Seamless connection with quality and regulatory processes enables proactive complaints handling, accelerating continuous innovation throughout the product lifecycle.
Vault Training manages training requirements to ensure compliance and role-based qualification for job and audit readiness. With centralized training records and a single audit trail, easily demonstrate compliance – always staying inspection ready.
Regulatory
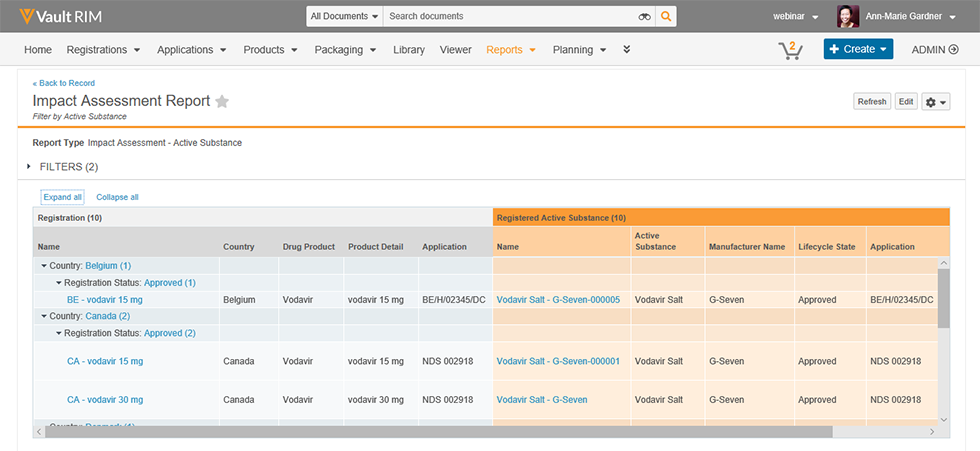
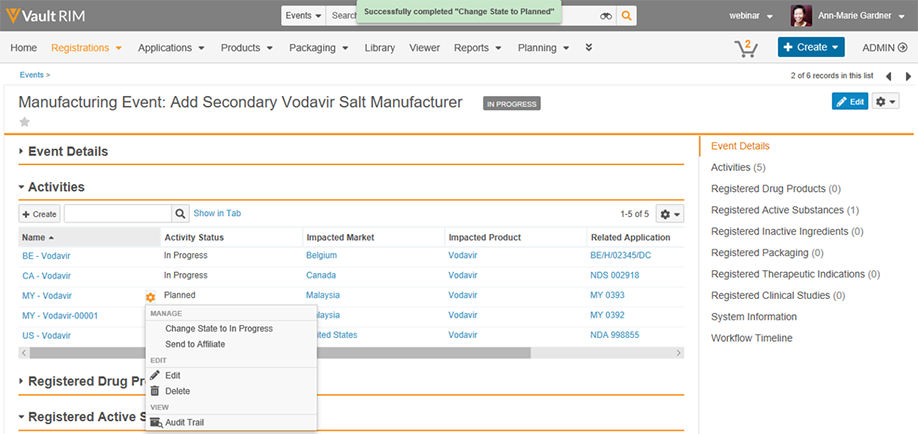
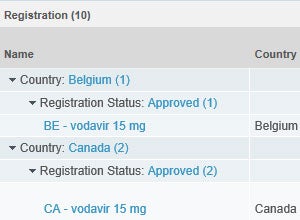
Vault Registrations provides a single global application for planning, tracking and reporting on product registrations and health authority correspondence and commitments. The improved visibility and data quality will streamline registration management and speed responses to health authorities.
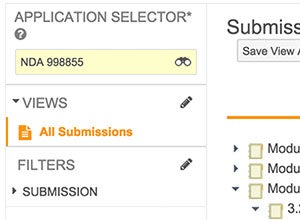
Vault Submissions manages planning through approval of all submission documents. Manual steps are automated throughout the process, from creating submission content plans, to rendering submission-ready documents, and tracking their status. Relationships between correspondence, commitments, submission records, and source documents, provide complete traceability for your regulatory activities.
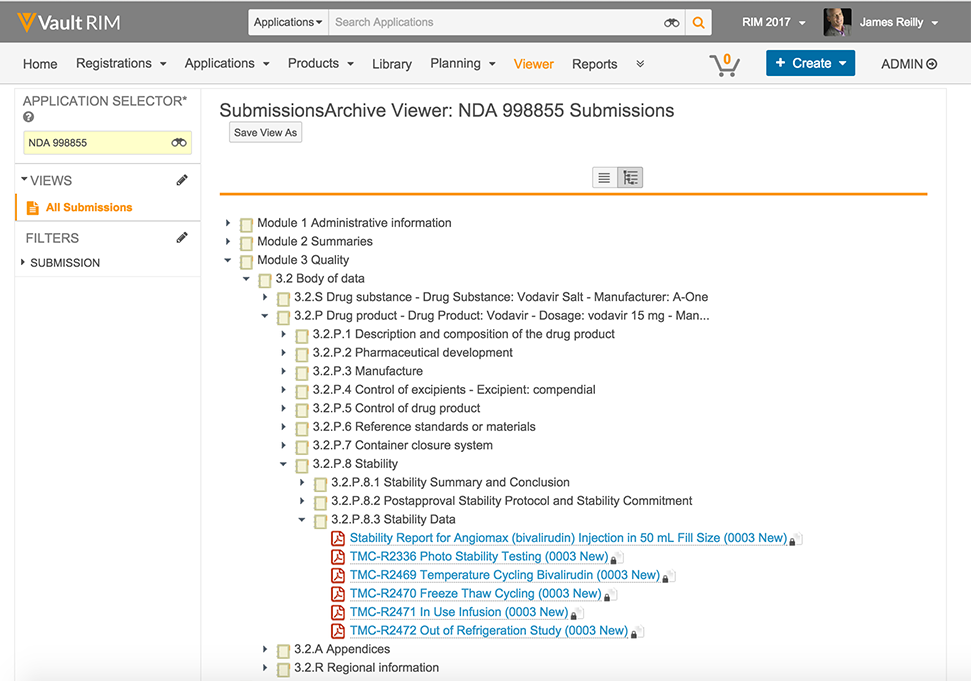
Vault Submissions Archive will store your complete history of regulatory submissions securely in the cloud. A high-performance cloud architecture makes access to published submissions fast and easy for authorized users. Affiliates can download submissions or submission components for reuse in local markets and upload their submissions to local health authorities.
Medical Content Management
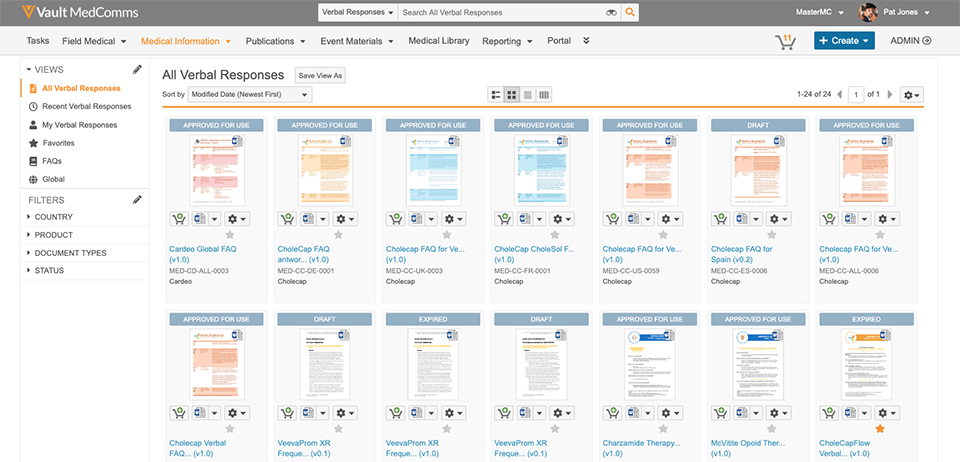
Vault MedComms provides a user-friendly global repository that simplifies collaboration and version control when managing all types of medical affairs content. The integration with a case management tool provides call centers with direct accesses to the most current information.
Commercial Content
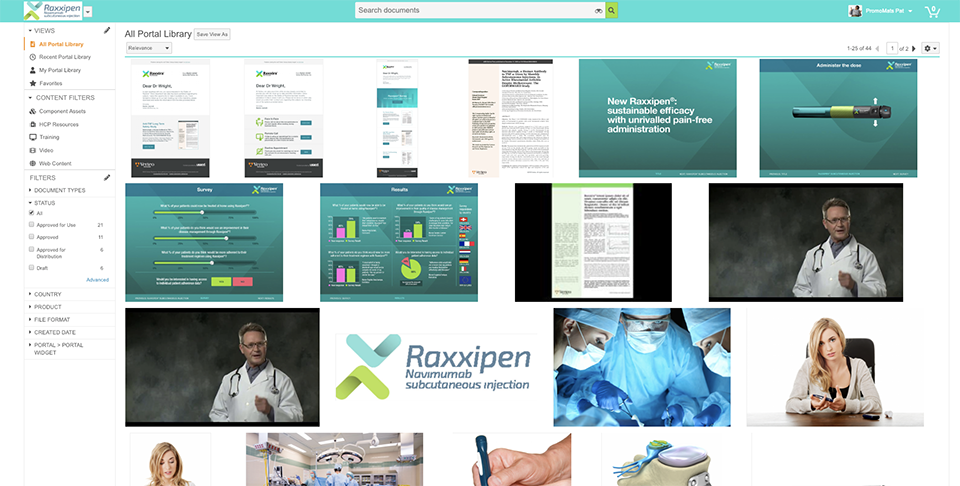
Vault PromoMats gets your message to market faster with easy collaboration, more efficient reviews, and single-click content distribution and withdrawal. Built-in digital asset management facilitates asset reuse, increases speed to market, and enhances compliance.
Veeva is a strategic partner to medical device and diagnostic leaders