Veeva Registrations
Plan, Track, and Report on Product Registrations
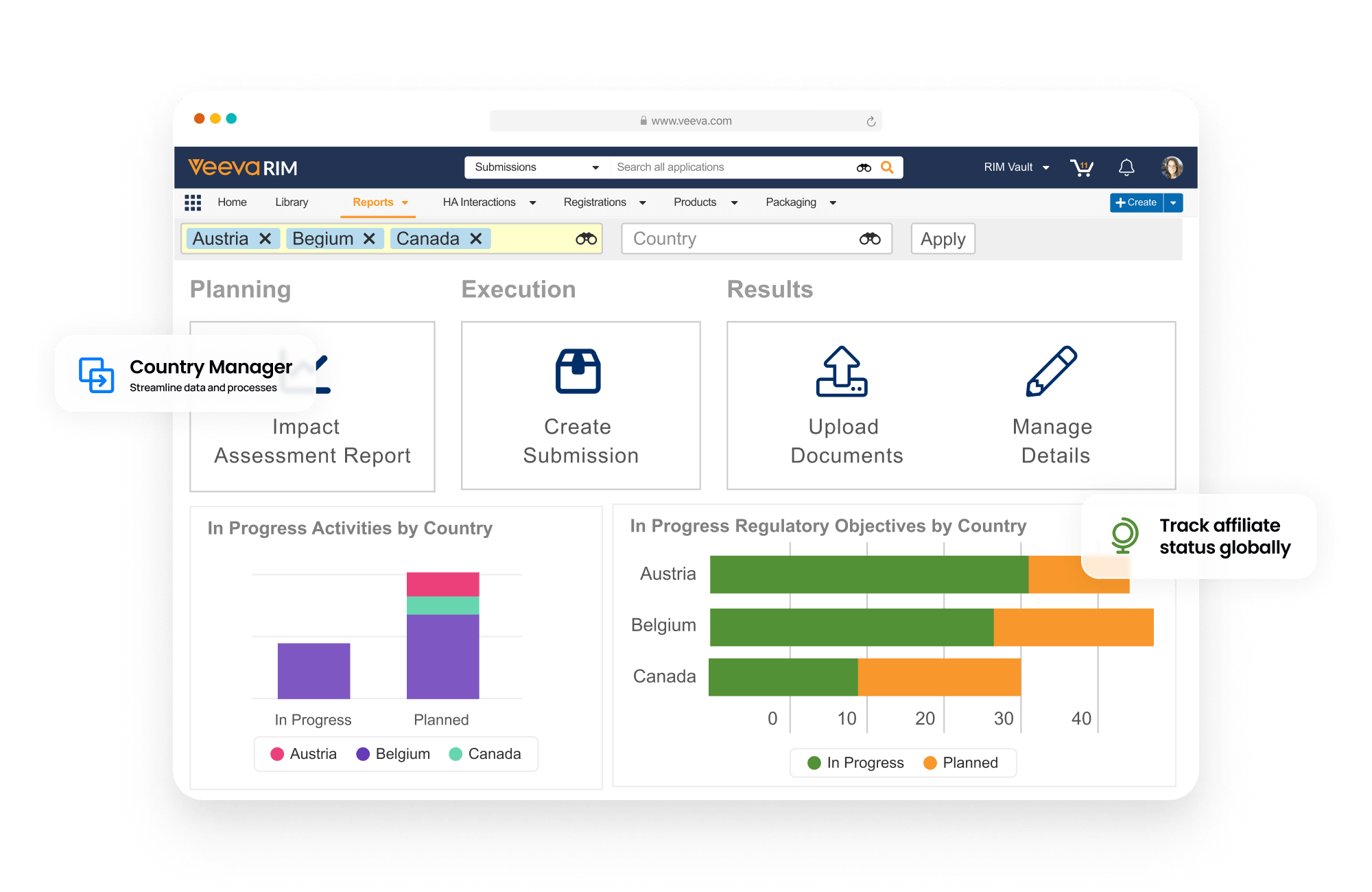
Registrations allows sponsors to plan, track, and report on global product registrations along with health authority correspondence and commitments.
Events provide the ability to manage product changes, from the initial assessment of the proposed change through submission creation, health authority interactions, and final registration update. Label changes can be tracked and managed at both the global and local level. Registrations also produces compliant product data output (e.g., xEVMPD and IDMP) for EU regulations.
Dashboards and reports allow personnel to track the progression of change events and provide an understanding of product registration locale.
Announced 2015 Status Mature Customers 100+
Learn how Veeva’s flexible data model supports the latest IDMP standards
Overview
Plan, Track, and Report on Product Registrations
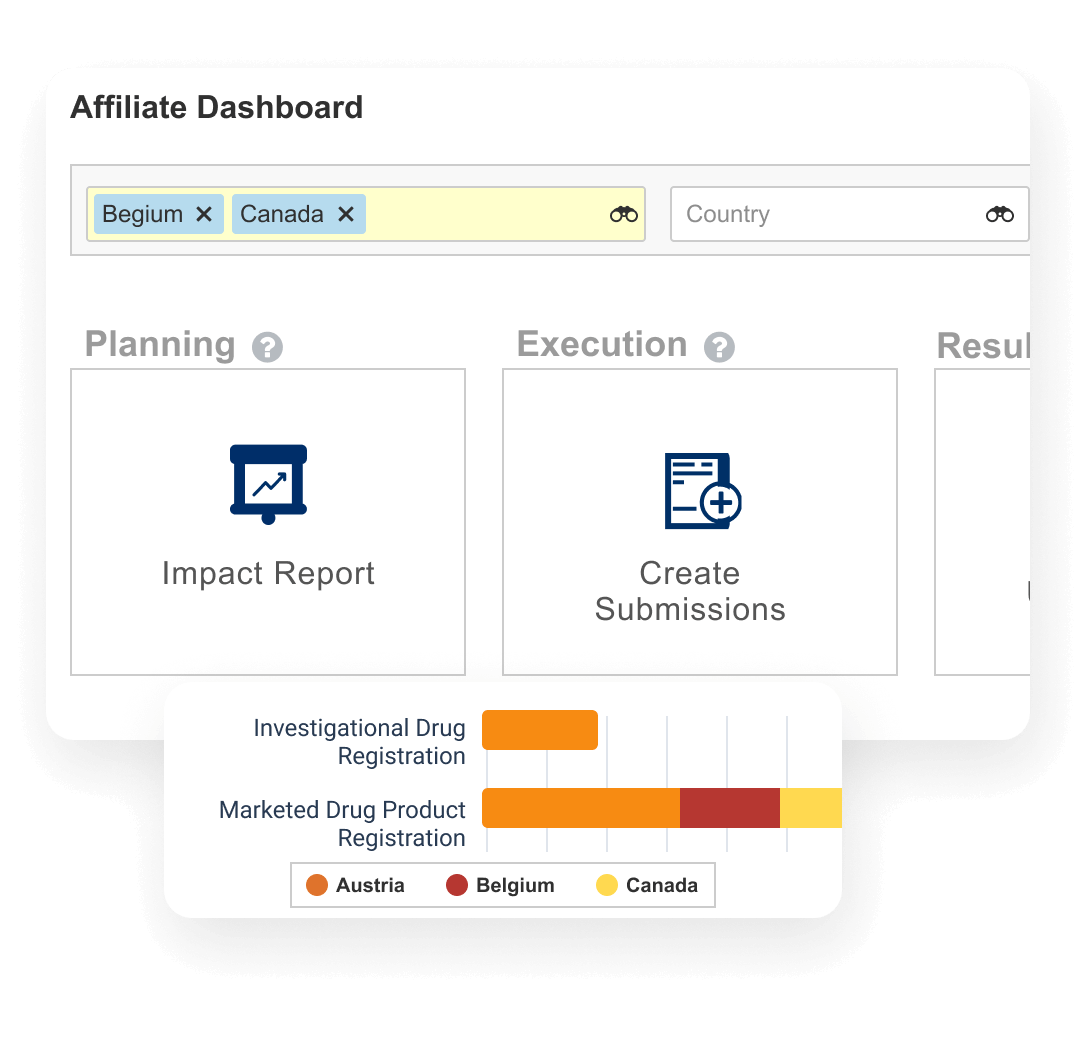
Registrations allows sponsors to plan, track, and report on global product registrations along with health authority correspondence and commitments.
Events provide the ability to manage product changes, from the initial assessment of the proposed change through submission creation, health authority interactions, and final registration update. Label changes can be tracked and managed at both the global and local level. Registrations also produces compliant product data output (e.g., xEVMPD and IDMP) for EU regulations.
Dashboards and reports allow personnel to track the progression of change events and provide an understanding of product registration locale.
Impact
Exceed the likely outcome
15
of the top 20 companies use Veeva RIM
88
IT systems consolidated into one
90%
reduction in written standards
Why Veeva Registrations
Plan, track, and report on product registrations
Customer Success
Veeva RIM is trusted by 400+
top and emerging biopharmas













