Veeva Site Connect
Simplify and Standardize Sponsor-Site Collaboration
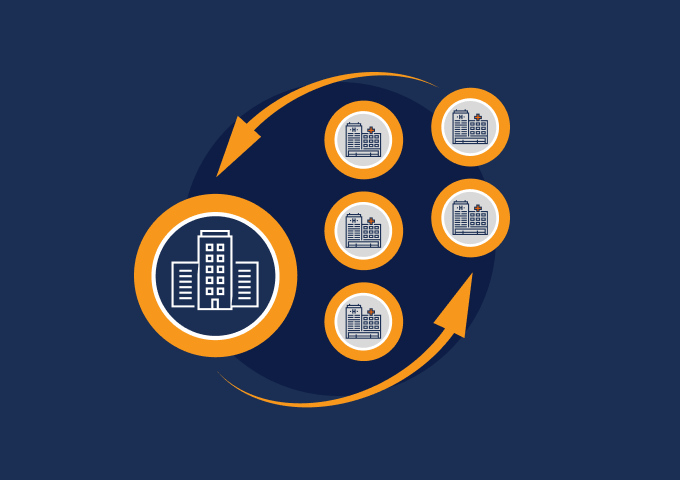
Site Connect allows sponsors and research sites to collaborate on a trial by automating the flow of information to and from sites during start-up, execution, and closeout.
Information flow includes protocols, essential document packages, study communications, safety reports, and payment letters. Required media is sent on closeout, including completed CRFs. Information sent and received is automatically filed in eTMF.
Research sites manage tasks, documents, and data in Site Connect. Optionally, sites can connect their SiteVault for enhanced functionality.
Announced 2020 Status Mature Customers 11-50
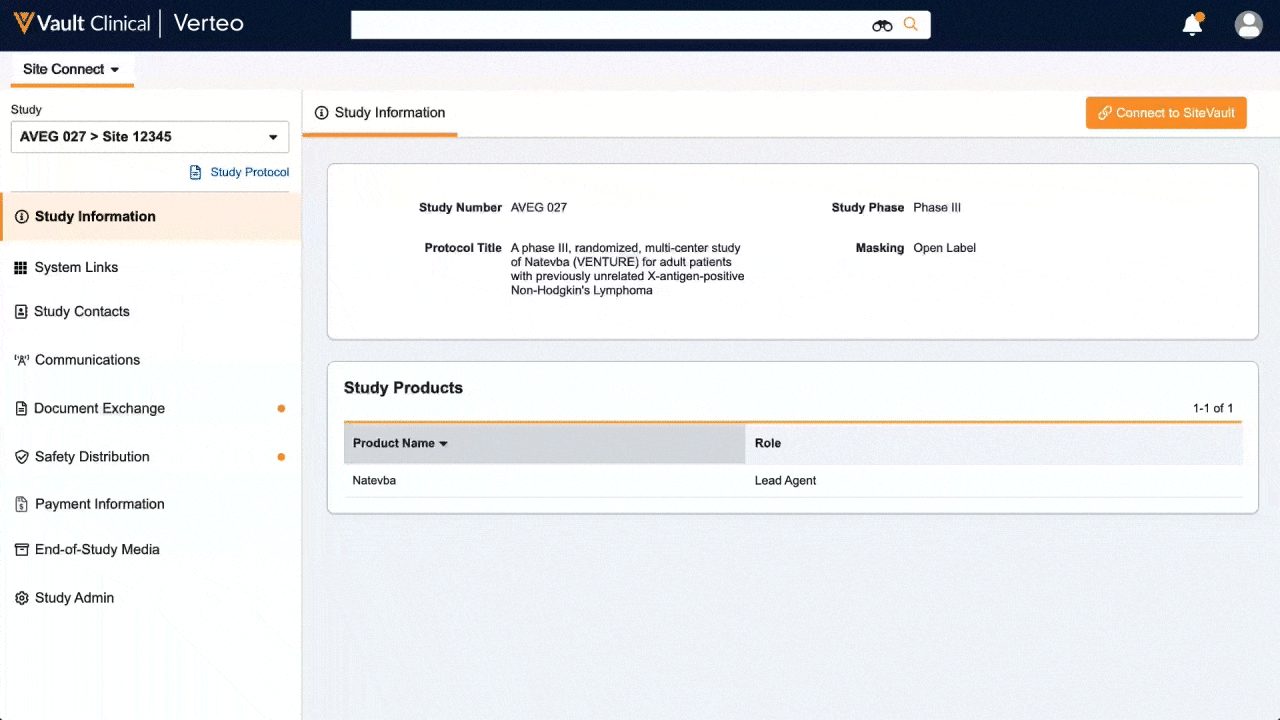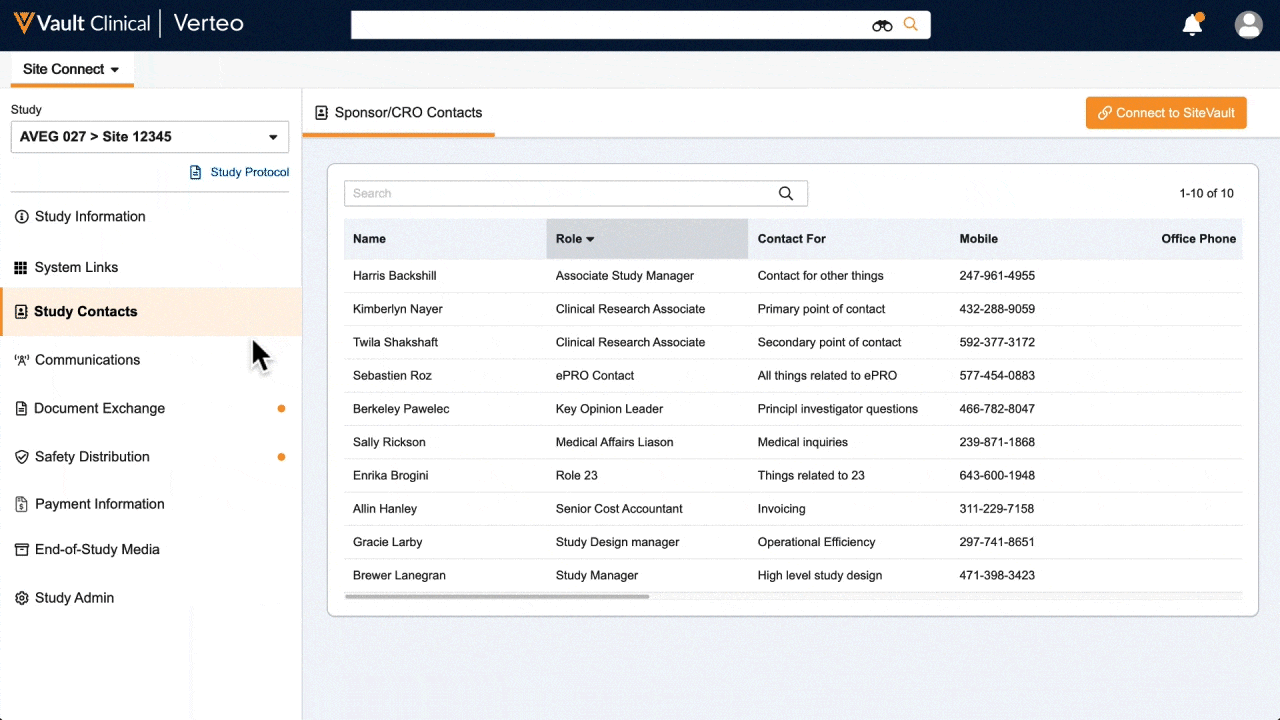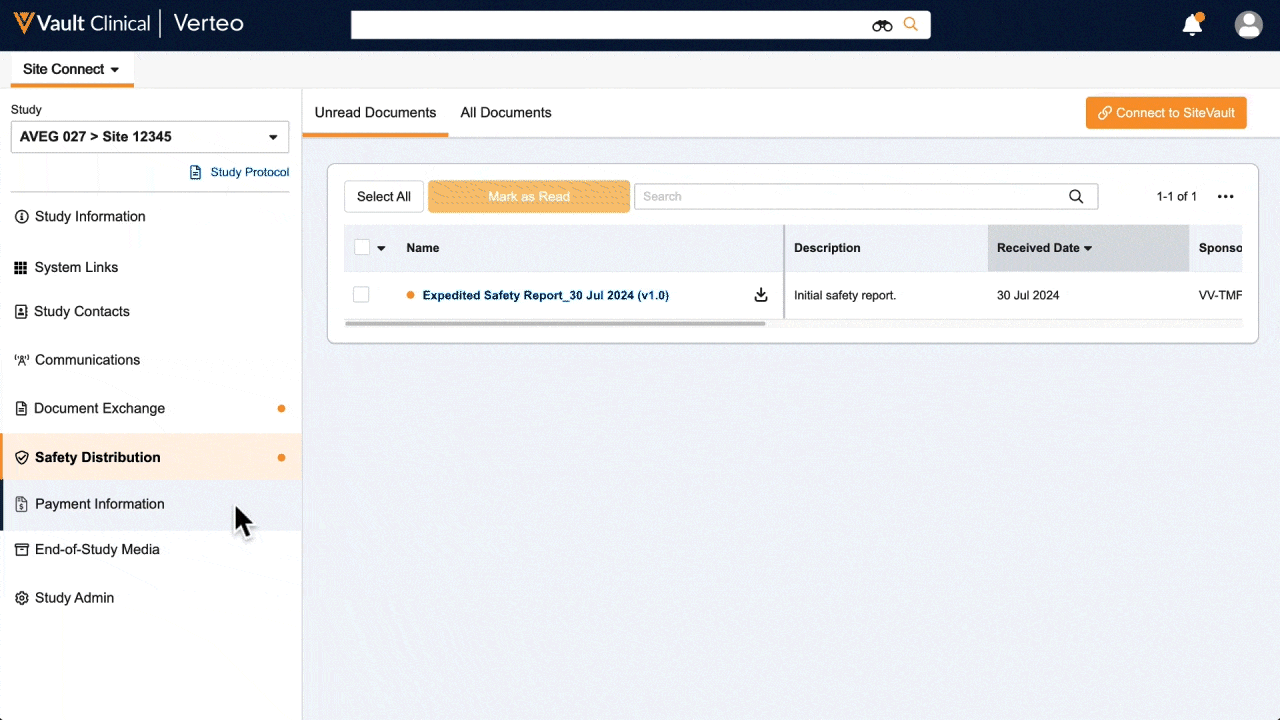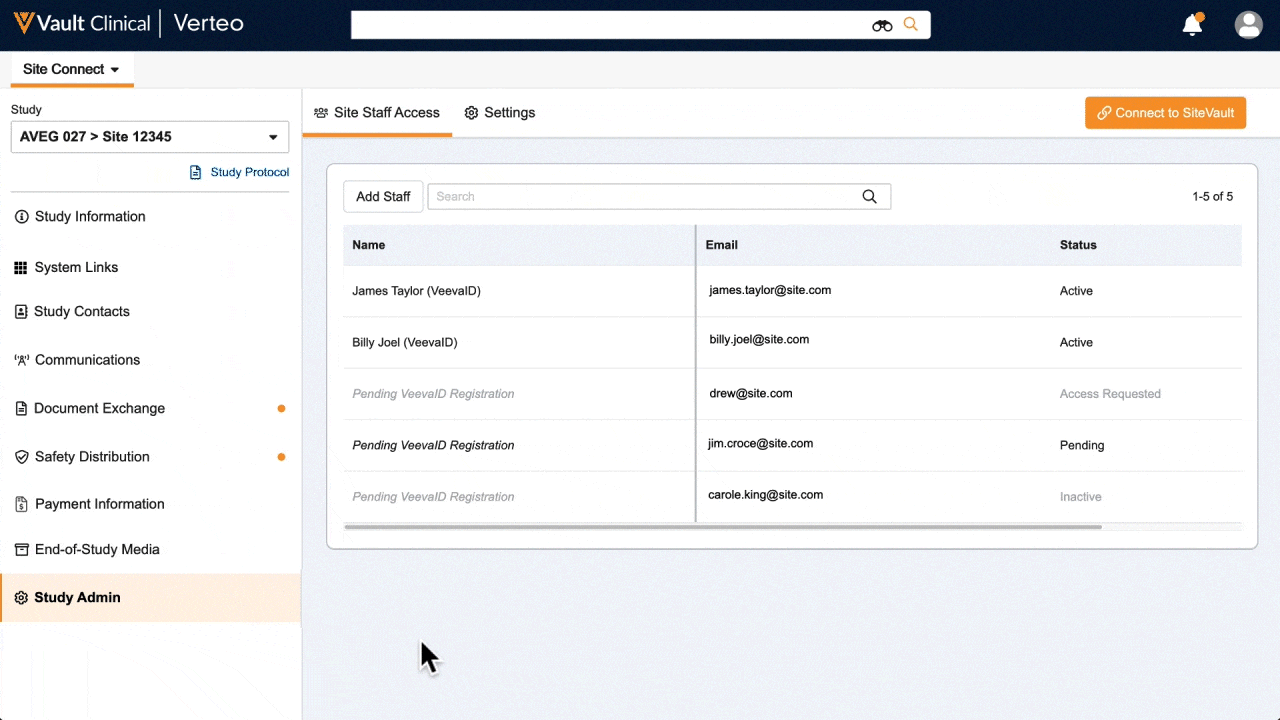
One place for document exchange, safety letters, study communications, payment information, and more.
See how AstraZeneca reduces manual safety letter distribution tasks by 70%
Why Veeva Site Connect
Preferred by sites
17K+
research sites use Site Connect
2,000+
site users log in daily
70%
reduction in site email volume






Overview
One place for sites and
sponsors to work together
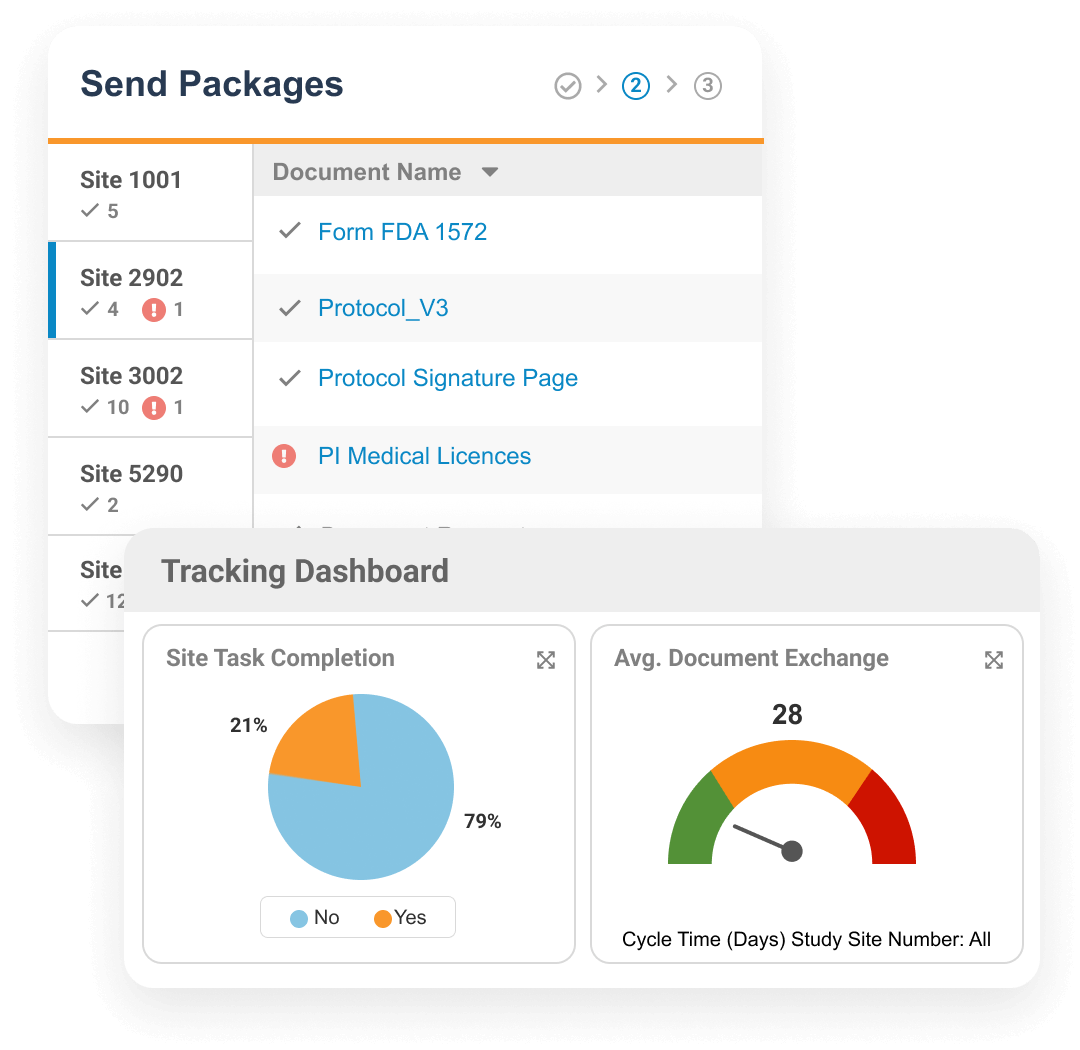
Veeva Site Connect allows sponsors and sites to collaborate in a single system while automating the flow of information from start-up to closeout.
Information flow includes protocols, essential document packages, safety reports, and payment letters. Required media is sent on closeout, including completed CRFs. Information sent and received is automatically filed in eTMF.
Research sites manage tasks, documents, and data in Site Connect. Optionally, sites can connect their SiteVault for enhanced functionality.

Impact
Accessible for all sites everywhere
Trusted by sponsors and CROs





Resources
Explore and Learn
Read Features Brief
Veeva Site Connect Features Brief
Watch Demo
Veeva Site Connect Explainer Video
Read White Paper
How Top Sponsors Build Strong Site Relationships That Last

Read eBook
Sites Share 5 Key Insights to Become a Sponsor of Choice

Read Blog
Q&A with Site: How Sponsors Can Better Support Sites

Watch Video
Bayer and a Site’s Vision for Simple, Standard Collaboration

Read Blog
How a Top 20 Pharma Engages Sites to Improve Trial Execution