Veeva EDC
The EDC for Everyone
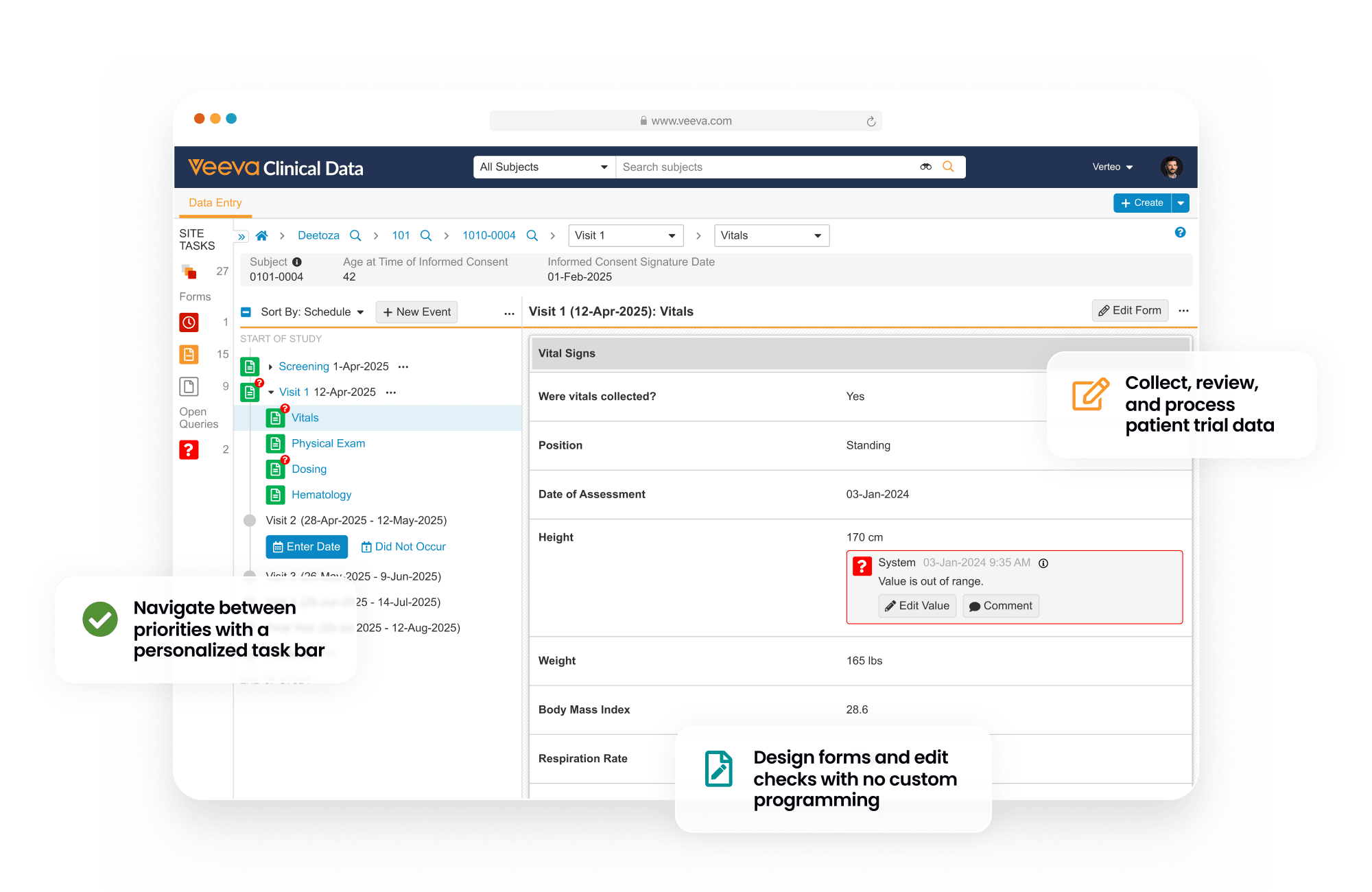
Electronic Data Capture (EDC) provides an end-to-end environment to collect, review, and process site-reported patient trial data.
During study start, EDC is used to design patient forms and quality control checks.
During study execution EDC collects all patient form data, local labs, and medical coding. Quality controls include querying, targeted source data verification (SDV) and protocol deviations. At the end of the study, EDC provides data lock and post-processing features, including automatic end of study media creation and archiving.
Announced 2016 Status Mature Customers 100+
Announcing Veeva eSource to eliminate paper and streamline data flow
Overview
The EDC for Everyone
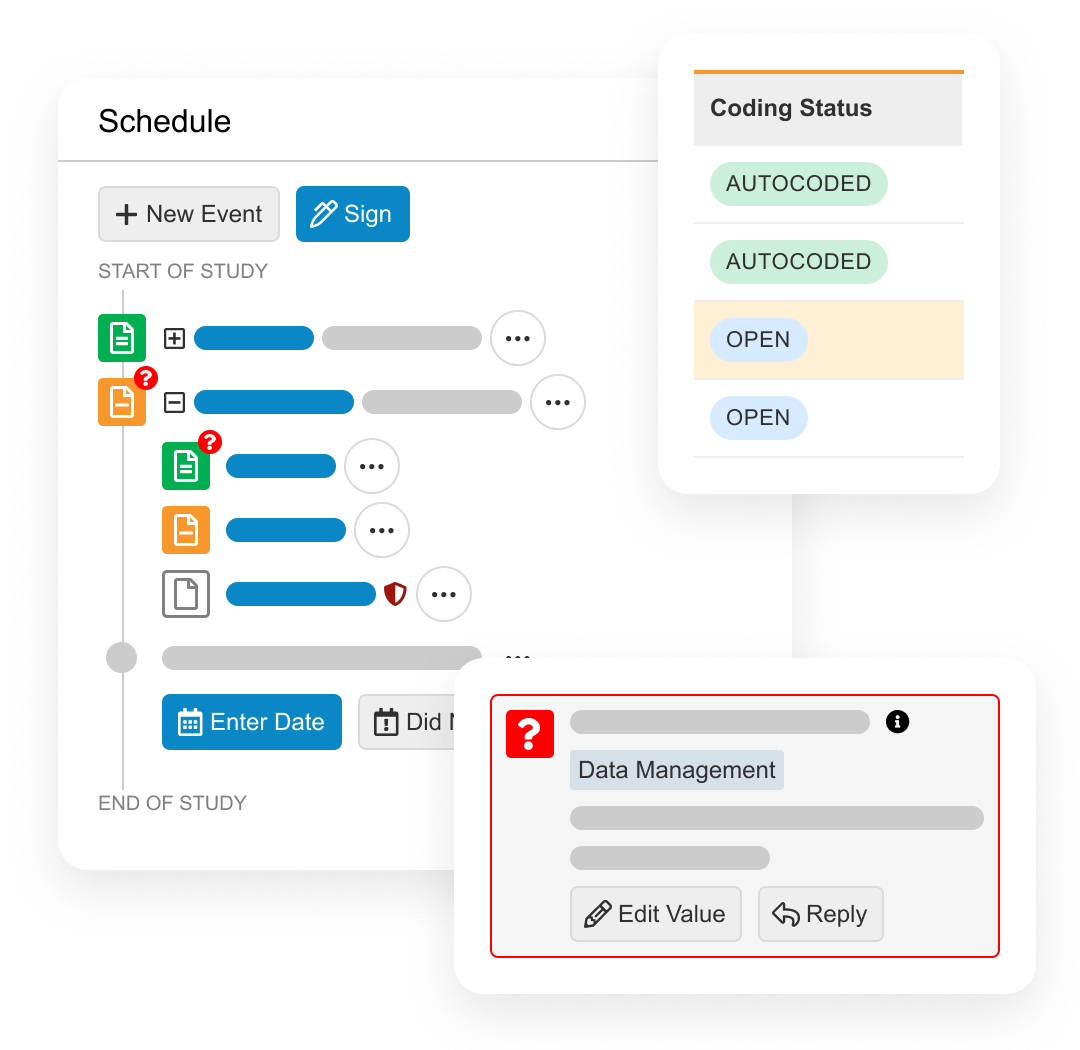
Electronic Data Capture (EDC) provides an end-to-end environment to collect, review, and process site-reported patient trial data.
During study start, EDC is used to design patient forms and quality control checks.
During study execution EDC collects all patient form data, local labs, and medical coding. Quality controls include querying, targeted source data verification (SDV) and protocol deviations. At the end of the study, EDC provides data lock and post-processing features, including automatic end of study media creation and archiving.
Impact
Proven value for complex studies
50%
faster study builds
100%
elimination of known custom functions
9x
faster to implement study changes
Why Veeva EDC
Modern EDC for agility and efficiency
Customer Success
Eight of the Top 20 biopharmas
switched to Veeva EDC










Resources
Explore and Learn
Read Features Brief
Veeva EDC Features Brief

Read White Paper
Eliminate Custom Functions with Veeva EDC
Learn More
Why Eight Top 20 Biopharmas Switched their EDC
Download White Paper
5 Hallmarks of an Agile EDC
Learn More
Meet the Sponsors and CROs Succeeding with Veeva EDC

Veeva EDC Demo Hub
See Veeva EDC in Action