Clinical Data for Modern Trials
Seamless and connected data flow
across patients, sites, and sponsors.
See how the connected data flow works
Veeva Clinical Data
Veeva Clinical Data brings together the core data collection and processing capabilities needed for a trial.
The clinical data applications are integrated to allow for one flow of data, ending up in a clinical database for aggregation and cleaning.
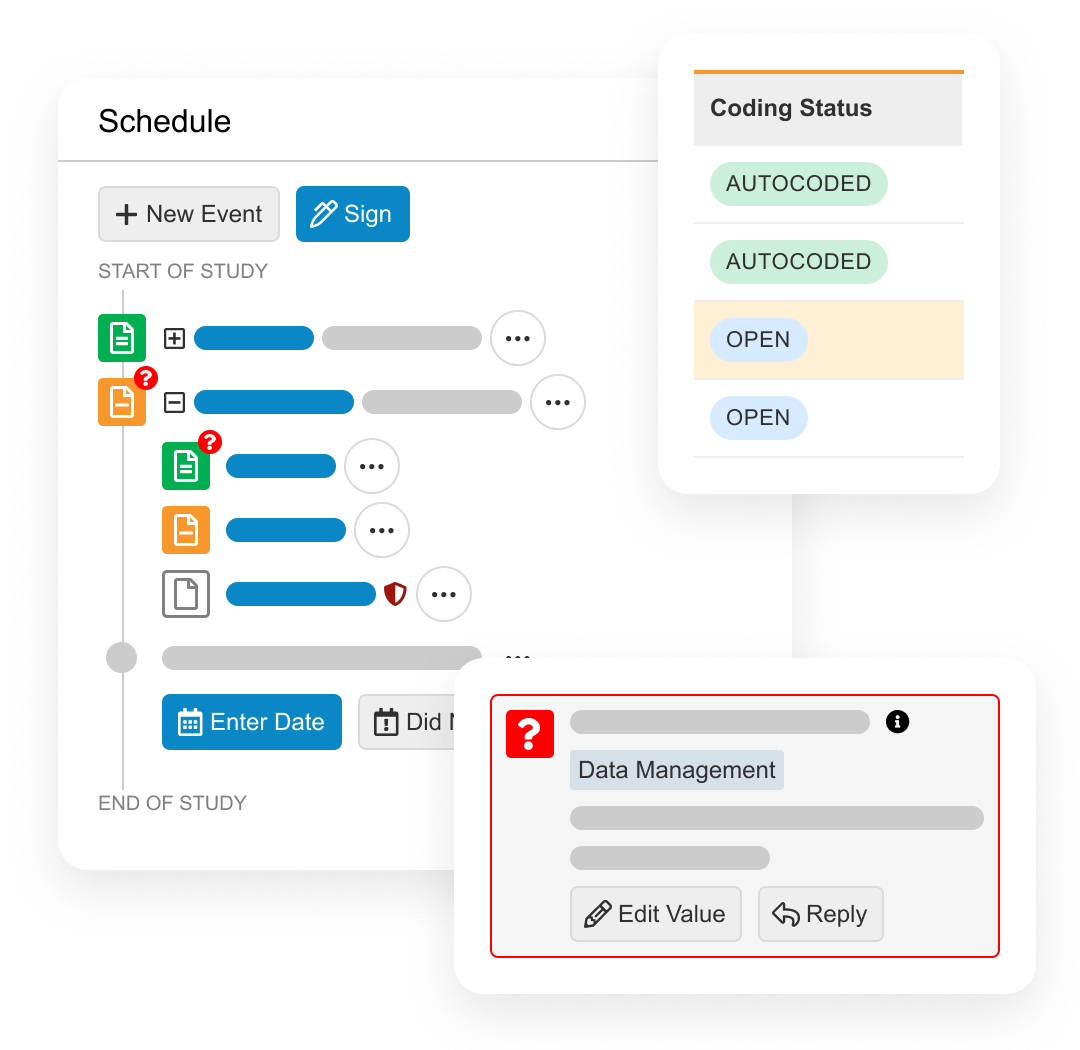
Veeva EDC
An electronic data capture application for sponsors to collect patient data from sites and ensure its quality.
Veeva CDB
A central environment to manage all data for a trial, including aggregating, cleaning, and transforming clinical data from multiple data sources.
Veeva eCOA
Captures questionnaire responses from patients, caregivers, and clinicians using an app or webpage, and provides sponsors an easy way to build surveys and distribute to sites.
Veeva Connections
Veeva Connections are Veeva-delivered integrations that seamlessly transfer data and documents between Vaults. For clinical data management teams, the Clinical Operations-EDC Connection provides up-to-the-minute site enrollment information. The Veeva RTSM-EDC Connection removes the need for duplicate data entry by sites and reduces associated reconciliation efforts by data management teams. The Veeva CDB-eCOA Connection transfers patient-entered data to reconcile with other data sources and identify trends. Visit the Veeva Development Cloud page for more Veeva Connections.
Impact
Innovation that Redefines Traditional Processes
Veeva Clinical Data fuels the pace and reduces the complexity of today’s clinical trials
50%
faster build cycle time
50%
less effort for execution and mid-study changes
50%
faster data cleaning cycle time
Customer Success
Customer Validation
Innovative companies are seeing the difference