When No News is Good News: How Recordati Passed a Recent Inspection with No Findings
0
findings in a recent inspection
25%
faster document ingestion
20+
manual trackers eliminated
Sponsors that fully or heavily outsource clinical trials are facing more complex compliance challenges under the new ICH GCP E6(R3) guidelines. These guidelines require structured and documented sponsor oversight processes and, importantly, a change in mindset about clinical trial execution and oversight in an outsourced setting.
Recordati is evolving its clinical operations model to address these challenges. As a global pharmaceutical company working in specialty primary care and rare diseases, Recordati is focused on developing new treatments and investing in medical innovations. In 2024, the company transitioned from fully outsourced to a hybrid operating model – bringing systems in-house and reviewing which activities CROs will perform in its internal systems. The goal was to improve data quality and monitoring, site engagement, regulatory compliance, and tracking of investigator-initiated studies in a centralized place with all other studies conducted on Recordati’s products.
Recordati and Veeva have been partners since 2016, and the Veeva Clinical Operations platform has underpinned Recordati’s transition to in-house systems across its portfolio, totaling up to 85 studies in 38 countries.
“Choosing Veeva was a natural solution because it allowed us to give CROs access to our systems,” recalls Luca Manfro, global R&D digital solutions manager at Recordati. Manfro joined Recordati in 2022 and has subsequently worked to adopt modern clinical operations solutions. “Today, we have nearly the full package, which we can use for both in-sourcing and outsourcing trials.”
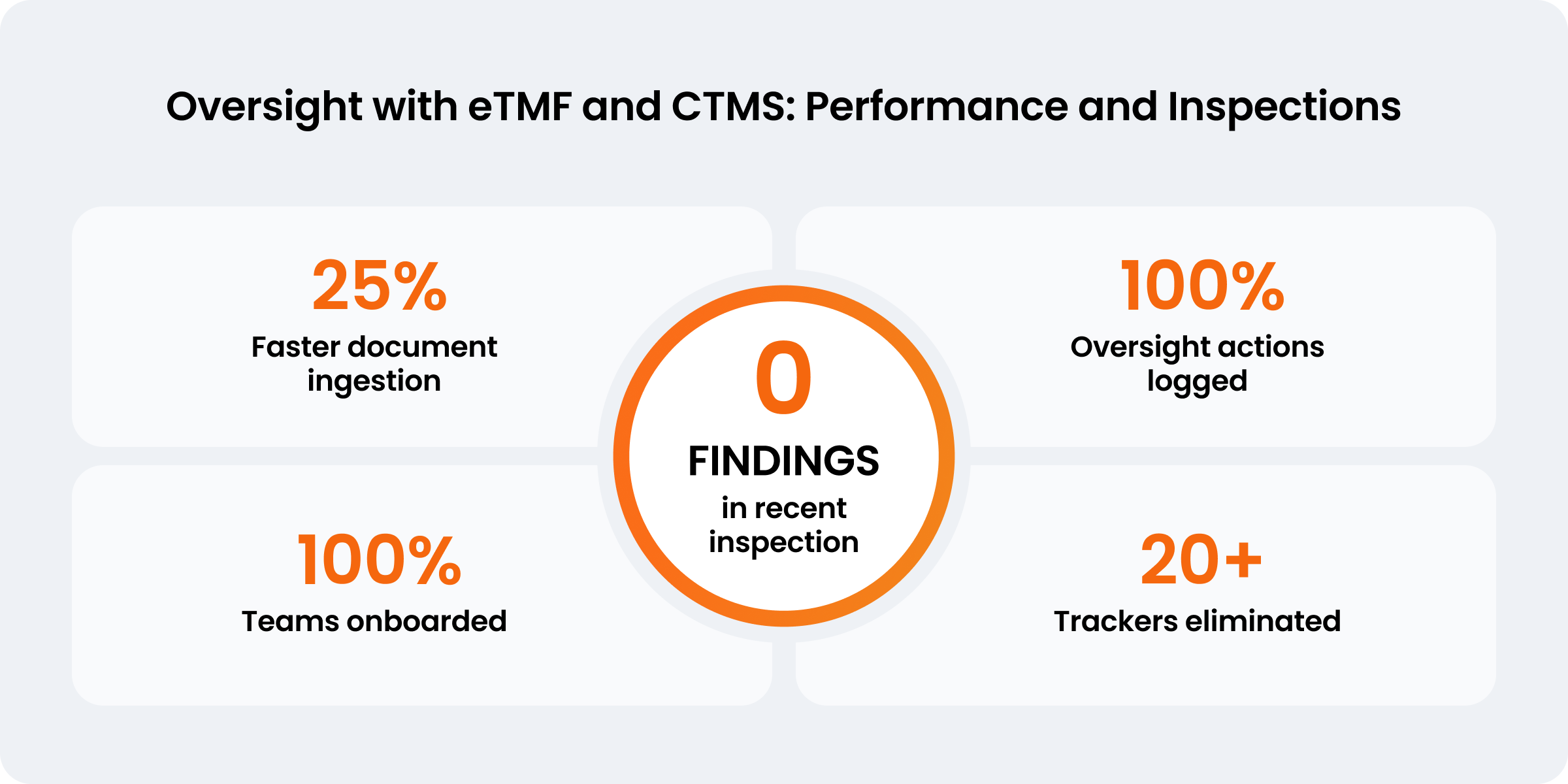
Since implementing the Veeva Clinical Operations platform, Recordati had no findings in a recent inspection. Manfro’s team has also eliminated over 20 trackers and reduced the document approval life cycle by 25%. “If an inspector says ‘show me what you have done about oversight,’ we can provide this evidence in seconds because everything is in the system,” Manfro explains.
The need for cohesive sponsor oversight
ICH E6(R3) introduced the concept of essential records, which includes documents and data, and makes the operational records generated by sponsors and CROs inspectable.
Essential records are used as part of the sponsor oversight or investigator supervision of the trial and should ensure:
- Tailored, fit-for-purpose risk-based oversight measures
- Quality assurance for trial activities done by investigators and providers
- Timely issue escalation and follow-up
Any disparity in sponsor and CRO systems makes oversight difficult. Separate vendor and sponsor SharePoints, meeting notes, emails, spreadsheet trackers, and phone calls all contribute to a complicated environment.
Recordati also faced several challenges when fully outsourcing clinical operations. For example, when employees left the organization, they did not leave records of decisions made or actions taken. This opened the company’s trials to regulatory risks and quality control problems that could slow down commercialization efforts.
Building oversight functionality
Under the new ICH E6(R3) guidelines, sponsors are subject to additional requirements for sponsor oversight, data governance, necessitating robust data management and monitoring systems to ensure data integrity and compliance with regulatory standards.
“When it comes to data governance, we can ensure better compliance by having everything tracked in our own systems: how we review operational data and control the entire management of our records and documents,” notes Manfro. “Without a centralized system like Veeva, it’s impossible to achieve good data governance and ensure quality,” he adds.
“Without a centralized system like Veeva, it’s impossible to achieve good data governance and ensure quality.” — Luca Manfro, global R&D digital solutions manager, Recordati
Aiming to increase its oversight of CROs to accelerate trials, Recordati added technology to standardize and automate processes. The company built on its existing Veeva eTMF foundation with Veeva CTMS. Over the years, traditional systems have evolved from managing trial execution to managing oversight for outsourced and hybrid studies. By bringing its CTMS in-house, Manfro’s team now plans, manages, and documents oversight actions through a central system encompassing tracking activities, milestones, specific user tasks, and decision-making.
This helps to demonstrate a risk-based oversight approach to inspectors and provides evidence that Recordati takes timely and appropriate actions to ensure patient safety and reliable trial results.
New systems meet new talent
Veeva CTMS offers centralized oversight functionality that allows Recordati to collect data efficiently, generate reports to assess status, and implement risk-based oversight planning. Manfro’s team can escalate issues, manage them effectively, and document decisions and actions across stakeholders throughout the study.
After integrating new technologies and processes, Recordati introduced roles like clinical trial liaison managers (CTLMs) and clinical trial application managers (CTAMs) to meet evolving needs.
CTLMs are senior CRAs who conduct oversight visits, verify data quality, and create reports based on SOPs, focusing on improving CRO accountability and identifying site performance obstacles. By working directly with sites, CTLMs develop and reinforce Recordati’s relationships at the site level and establish whether nurses are trained appropriately, supplies are correct, and that pharmacies are properly stocked, for example. “CTLMs are crucial. They provide a comprehensive assessment of the CROs’ effectiveness at the site and are a key component of sponsor oversight,” says Manfro.
CTLMs develop co-monitoring and oversight visits in Veeva CTMS, and oversight documents are stored with sponsor-only access. They can manage contact lists, monitor reminders for study plan updates and milestones, and record any significant actions or decisions.
Manfro sees several key improvements from stronger oversight, including:
- Process digitization and automation
- Reduced error rates during study start-up
- Faster issue resolution
- Improved collaboration with sites and CROs
- Better traceability and documentation
- Accessible and editable data throughout the trial
- Greater inspection readiness
- Strengthened sponsor relationships at the sites
Vision for a connected clinical environment
Manfro has many plans for the future Recordati clinical system environment. The goal for the next trial is to invest in more internal systems that simplify and standardize operations, such as Veeva eCOA and Veeva Study Training. “Having Veeva as our clinical partner has improved our data quality and inspection-readiness,” says Manfro. “More oversight is a change of mindset for sponsors and CROs. We need to invest in a continuous oversight improvement process, which is not a nice-to-have but a must in light of ICH E6(R3).”
“More oversight is a change of mindset for sponsors and CROs. We need to invest in a continuous oversight improvement process, which is not a nice-to-have but a must in light of ICH E6(R3).” — Luca Manfro, global R&D digital solutions manager, Recordati
Learn more about the sponsor oversight capabilities of Veeva CTMS.




