eBook
The New Rules of Pharma Events:
Handbook for Success in the Hybrid Era
Handbook for Success in the Hybrid Era
Events: Make-or-break link in the customer journey

Figure 1: GSK integrates its global events strategy with the customer journey (watch video)
Over the last two years, the way pharma companies run events for healthcare professionals (HCPs) underwent two significant transitions. First, they went from mostly face-to-face to exclusively virtual out of necessity during the pandemic; second, they went from entirely virtual to the new hybrid era, out of choice. And while digital events have increased 600% relative to pre-pandemic,1 in-person events are back and highly valued, especially for providing education and support to HCPs.
When assessing event effectiveness in the new hybrid model, it’s important to consider which customer interactions occur before, during, and after an event across multiple channels [Figure 1]. Yet, the traditional way of managing events—using spreadsheets, highly customized horizontal platforms, or proprietary technologies—makes it difficult to bring event data together with insights from other activity channels.
By treating events as a separate channel, rather than as an integrated touchpoint in the customer journey, your team could miss out on valuable customer interaction data from one of the highest impact touchpoints with HCPs.
This handbook details the success factors for an impactful global events model. Events strategies succeed when teams focus on the metrics that matter. To offer a truly omnichannel customer journey, companies need to consider event activities within the wider mix of HCP engagement channels. Only then can your commercial team determine whether your events drive strategic outcomes for the brand.
Single view of all engagement activity
In the past, most events were in person, with speakers, reps, and attendees gathering in the same place. They were organized as standalone interactions and relied on email, or even paper-based, invitations. Field teams tracked engagement activities (such as rep emails, in-person visits, and virtual calls) in CRM software but kept event data in a separate silo, resulting in an incomplete customer view.
Meanwhile, the event data they collected typically focused on attendance: the number of attendees, cancellations, and response time to invitations. Companies lacked understanding of the customer interactions happening before, during, and after an event across other channels.
With 58% of HCPs saying they prefer virtual or hybrid medical conferences,2 pharma companies have an opportunity to expand the type of event data they can collect, and insights they can gain, into an HCP’s engagement with a specific brand.
When you integrate events into your overall engagement strategy, information from each customer touchpoint builds upon the last interaction. You can combine data from all your event types with insights from other activity channels—face-to-face visits, virtual meetings, phone calls, etc. Sales and marketing teams then gain complete visibility into field activities and results, which they can use to personalize interactions.
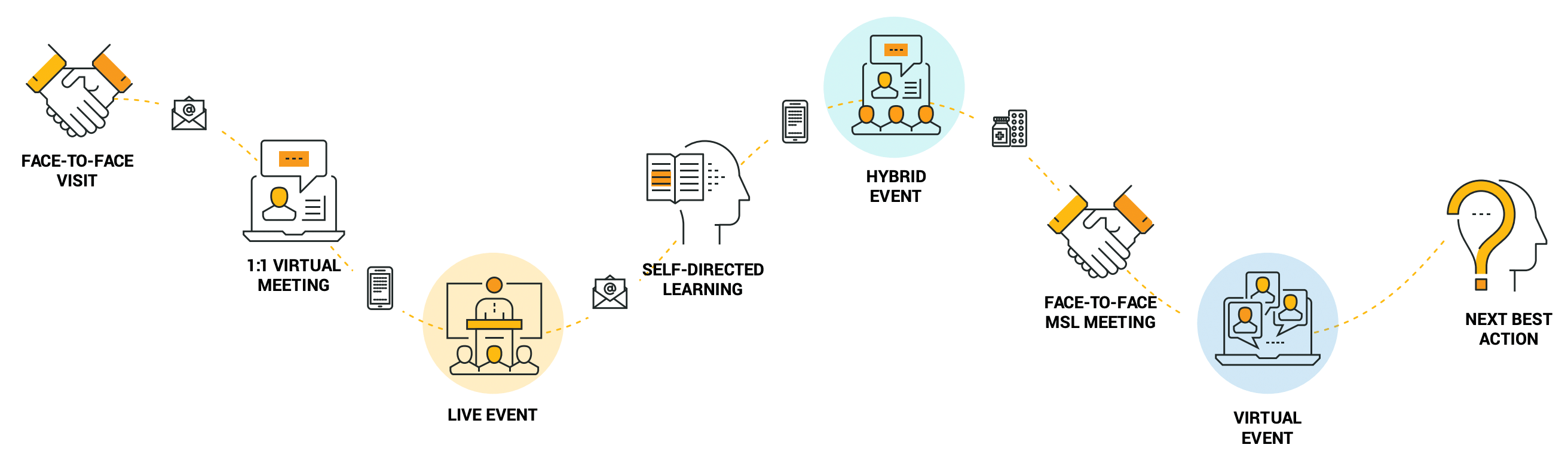
This integrated view of all customer activities and affinities makes it easier to plan omnichannel campaigns and execute more effective events across the right mix of event types [Figure 2].
Figure 2: Impactful events inform field teams’ next best action with HCPs
Boehringer Ingelheim: Event data fuels global customer insights
With more than 50,000 employees, Boehringer Ingelheim is one of the world’s largest pharmaceutical organizations, renowned for developing innovative treatments.
Given its size, harmonization across its 88 regional offices is critical to the commercial team’s operational efficiency and success. However, regional teams lacked a global, integrated event solution and were accustomed to using their own separate solutions. This resulted in a fragmented landscape, without a standard planning process or consistent reporting.
The team decided to roll out Veeva CRM Events Management across 77 countries over three years, providing local teams with a technology solution that is fully integrated with their CRM and is both secure and compliant.
Instead of constant back and forth over email to propose events and share bits of information slowly over time, Boehringer Ingelheim streamlined event planning across the company. Event organizers now use CRM Events Management to reserve dates, manage invitations, plan budgets, get approvals, and more, while meeting regional regulatory requirements.
Using the deep insights available in its new event solution, Boehringer Ingelheim can also deliver the right mix of in-person, virtual, and hybrid events. While face-to-face meetings previously dominated event schedules, the company currently offers about 80% of its total events as virtual offerings.
Outcomes with Veeva CRM Events Management

Hybrid era success factors
In the hybrid era, a successful event simultaneously provides value to HCPs while furthering the goals of the brand. This level of customercentricity requires pharma companies to know what has worked for HCPs in the past, what would deliver the most value at the next touchpoint, and the business impact of choosing one type of event format or content over another.
To do this, you need best-in-class technology, event data that can be embedded into CRM, and processes that allow insights to flow to the right people within the organization.
Technology: Deliver on the promise of omnichannel
One thing leading organizations have in common is best-in-class technology. The right events management software can deliver on the promise of omnichannel by providing HCPs with a seamless customer experience across the channels they prefer.
In the past, some companies customized or stitched together various internal systems to match the workflow of each unique event type. Others relied on their logistics partners’ software, even though it wasn’t designed with customer data management in mind. These methods were time-consuming and expensive, resulting in multiple applications that couldn’t share data.
The right technology should serve all event types, delivering better visibility with greater compliance [Figure 3]. The application should be pre-integrated with engagement data across all other channels to achieve a complete view of your customers. Using a common data architecture can be a powerful and cost-effective way to minimize the resources for linking data.
Figure 3: Five success factors for Events Management software
COMMERCIAL IMPACT
- Cost-effective, trackable, and quick for events teams to use
- Open so logistics partners can access
ACTIONABLE
- Measure outcomes
- Pinpoint the next steps for each HCP
SCALABLE
- Maintain budgets and countryspecific regulatory requirements
- Enforce global standards
INTEGRATED
- Pull insights from other event planning tools (e.g., preparing for medical congresses), providing coverage of different potential touchpoints with HCPs
REAL-TIME
- Easy and quick to pull compliant, real-time insights along the customer journey from data captured
If a rep and an HCP have a face-to-face meeting, that rep should be able to track the HCP’s reactions to the content and key messages shown and record the customer’s channel preferences in a call report. This information should be quickly accessible to the events team, who can use it to shape the format and content of future events.
It should also be easy to integrate other third-party solutions (for example, those used by external congress managers) to facilitate the exchange of information between field teams and external partners.
DID YOU KNOW?
Over one million events run annually through Veeva CRM Events Management across 100+ customers.
"We had a clearly defined vision to delight all event customers. Veeva CRM Events Management was a critical delivery mechanism for this vision, as it allowed harmonization as opposed to standardization." Dave Yates, Global Product Director, GSK
Data: Understand how customers interact with the brand
To deliver a coordinated omnichannel experience, information from each customer touchpoint must build upon the last. This requires a careful appraisal of how actionable the data you’re capturing at events is to the broader company.
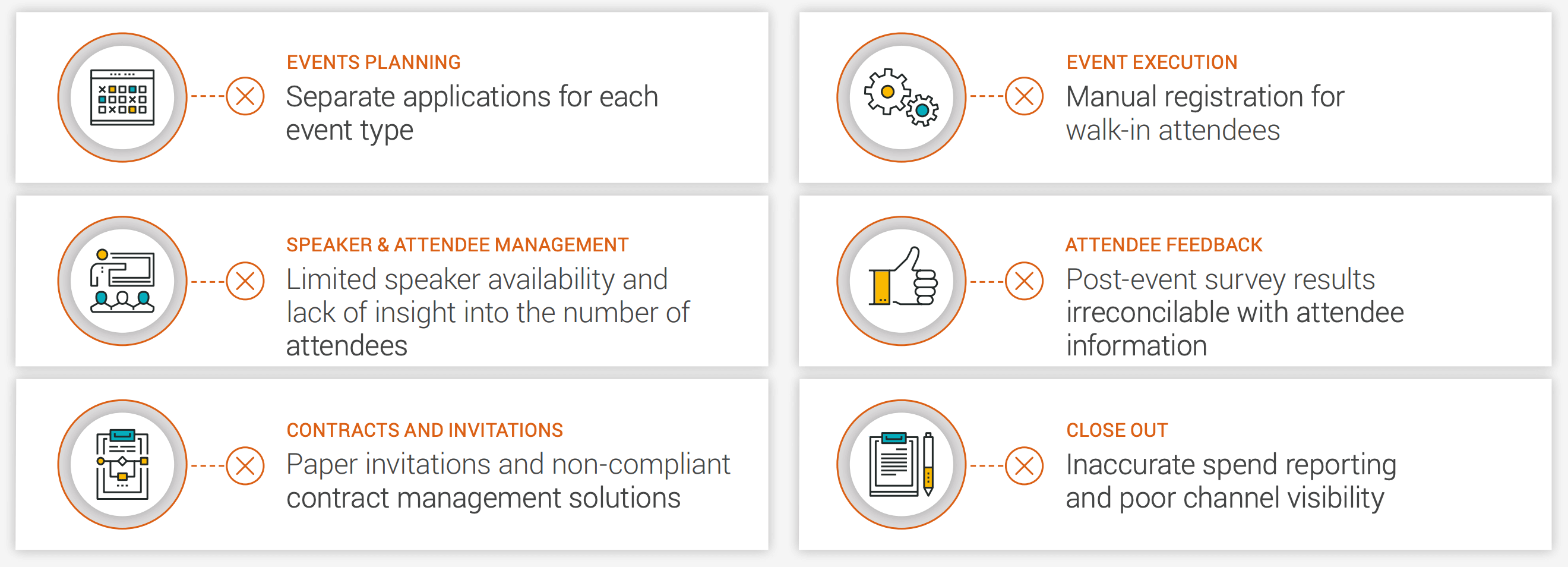
Consider speaker programs, for example [Figure 4]. Your events team might only gain access to relevant data several weeks after the event. Sales, medical, and marketing teams might lack the context they need to determine the next action. If you have an incomplete view of the HCP’s journey with your brand, it can be difficult to pinpoint which rep- or MSLdriven action led to specific HCP behaviors.
Figure 4: Symptoms of a traditional speaker program
If you want to deliver true omnichannel, you have to measure and manage events like any other HCP engagement channel. Avoid relying on surface-level KPIs (e.g., registration, attendance, and cancellations). The goal should be to generate deep customer insights to engage HCPs in the most effective, promotionally responsive way.
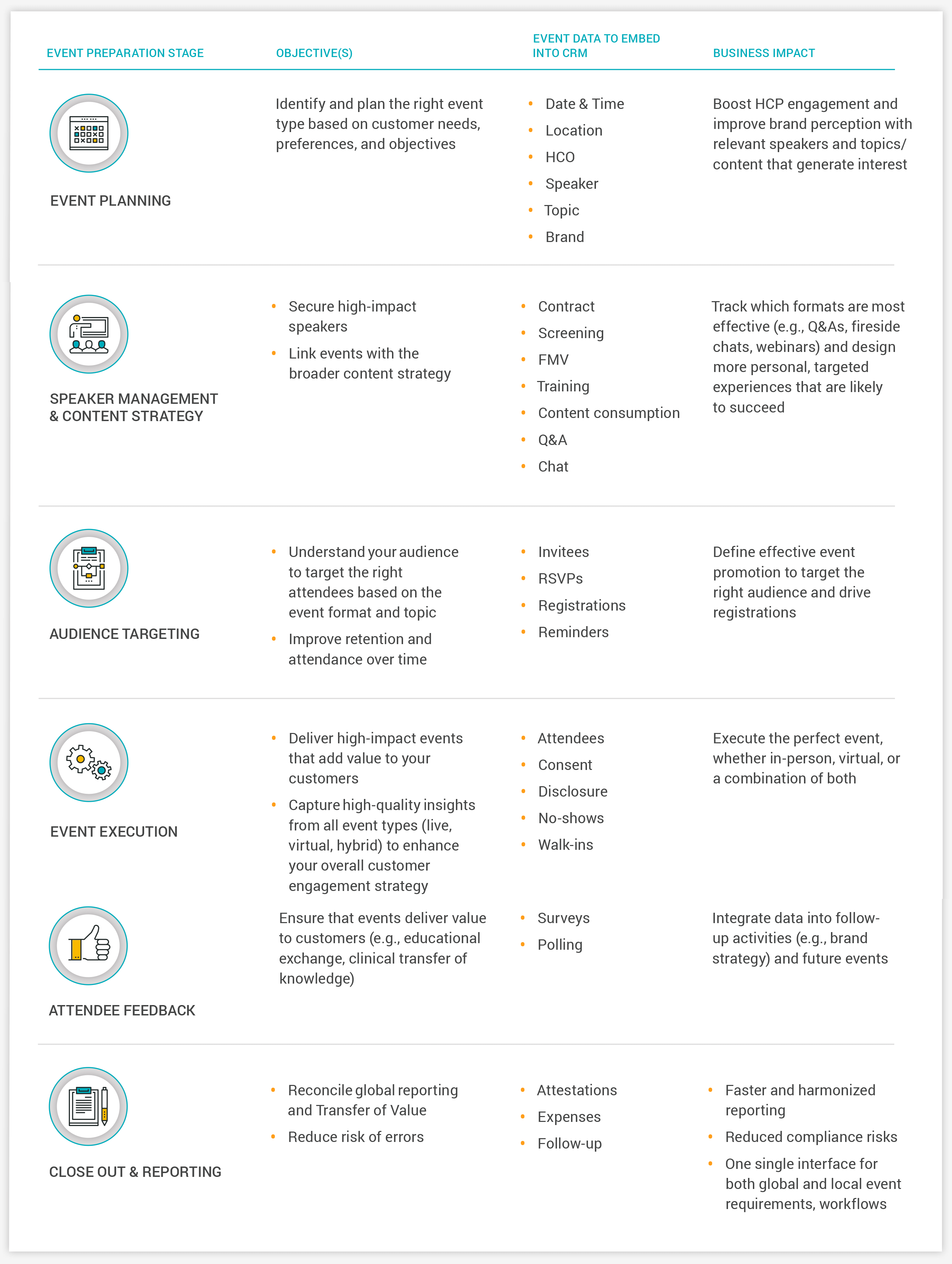
Event data is most powerful when integrated with channel activity data, so insights can be tied back to individual HCPs. For this, your team needs clean and consistent data that combines rep activity, content, and channels—including events—into a single solution [Figure 5].
Sales, medical, and marketing can then all use the same HCP-centric data to inform the commercial strategy. Event data and content become drivers of business goals, not just standalone metrics.
Figure 5: Integrated events have measurable business impact
Processes: Insights flow to those who need to know
With so much new data at your fingertips, your teams will need to evaluate business processes to avoid a scenario where the volume of information is an obstacle to taking action.
Start rethinking these processes by asking yourself two questions:
- Insight vs. information. Is the data we’re capturing nice-to-know information or insight that can determine the next actions for each HCP?
- Platforms and processes. Are our business units using consistent platforms and processes or is there variation across (and even within) them?
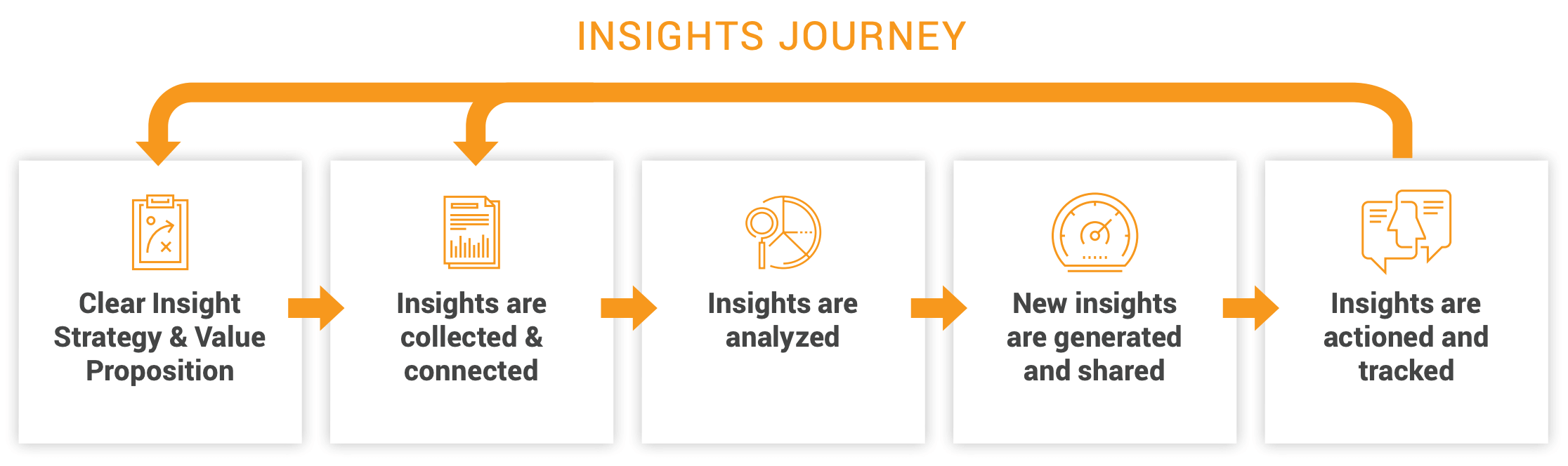
The right approach starts by defining the insight strategy upfront, which helps to set and measure organization-wide KPIs at each touchpoint, including events [Figure 6].
Next, consider which platforms and processes are already in place across the business to ensure there are no information asymmetries as insights are collected and connected. Best-in-class technology is a prerequisite at the third (analysis) stage to filter information regularly and pinpoint recent accurate insights.
At the insight-sharing stage, set up your platform to funnel the right insights to the right users within the organization. Key stakeholders like sales, medical, and marketing should be able to access and use event insights as part of the overall engagement strategy. Finally, once teams have acted on insights and are tracking progress, they should be able to funnel feedback to the start of the process to inform the ongoing brand strategy.
Figure 6: Insights are filtered and fed back to teams
"We want to understand our customers better so that we can adapt our offerings. And that’s exactly what Veeva CRM Events Management offers: the ability to generate insights so that we can better manage and support our customers." Beate Hansmann, Global Capability Owner, Meetings and Events, Boehringer Ingelheim
Conclusion
It’s taken a few years of digital-only HCP interactions for pharma companies to realize that events should be considered part of the HCP customer journey, just like channels such as emails or meetings. As a result, many now realize that collecting and measuring event data in a separate silo from all other activity data doesn’t make sense.
The next step is to bridge the gap and gain one single view of all your interactions with an HCP. Your team may see the CRM system as where field rep activities are housed. While that’s true, it’s also a gold mine of valuable insight about HCP behaviors and preferences that are core to omnichannel.
Connecting these activities, and effectively sharing these insights with the stakeholders who need to know, will help personalize the next interaction, whether that’s an event, speaker program, or one-to-one meeting. This integrated approach to the customer experience is needed to drive success in the new hybrid era.
About us
Veeva is the global leader in cloud software for the life sciences industry. Committed to innovation, product excellence, and customer success, Veeva serves more than 1,100 customers, ranging from the world’s largest pharmaceutical companies to emerging biotechs.
As a Public Benefit Corporation, Veeva is committed to balancing the interests of all stakeholders, including customers, employees, shareholders, and the industries it serves. For more information, visit veeva.com/eu.
1 Veeva CRM Engage for Events, April 2021–April 2023
2 G-Med, Q4 2021 poll of 1,206 HCPs across 75 countries: “Which medical conference format do you prefer?” (PharmaForum)
