eBook
Building the Future of MLR with AI: Fastest Path to Approved Content
AI’s value is crystallizing as part of a bigger opportunity to retool end-to-end content operations. Learn what process improvements support AI’s success and keep MLR review in focus.
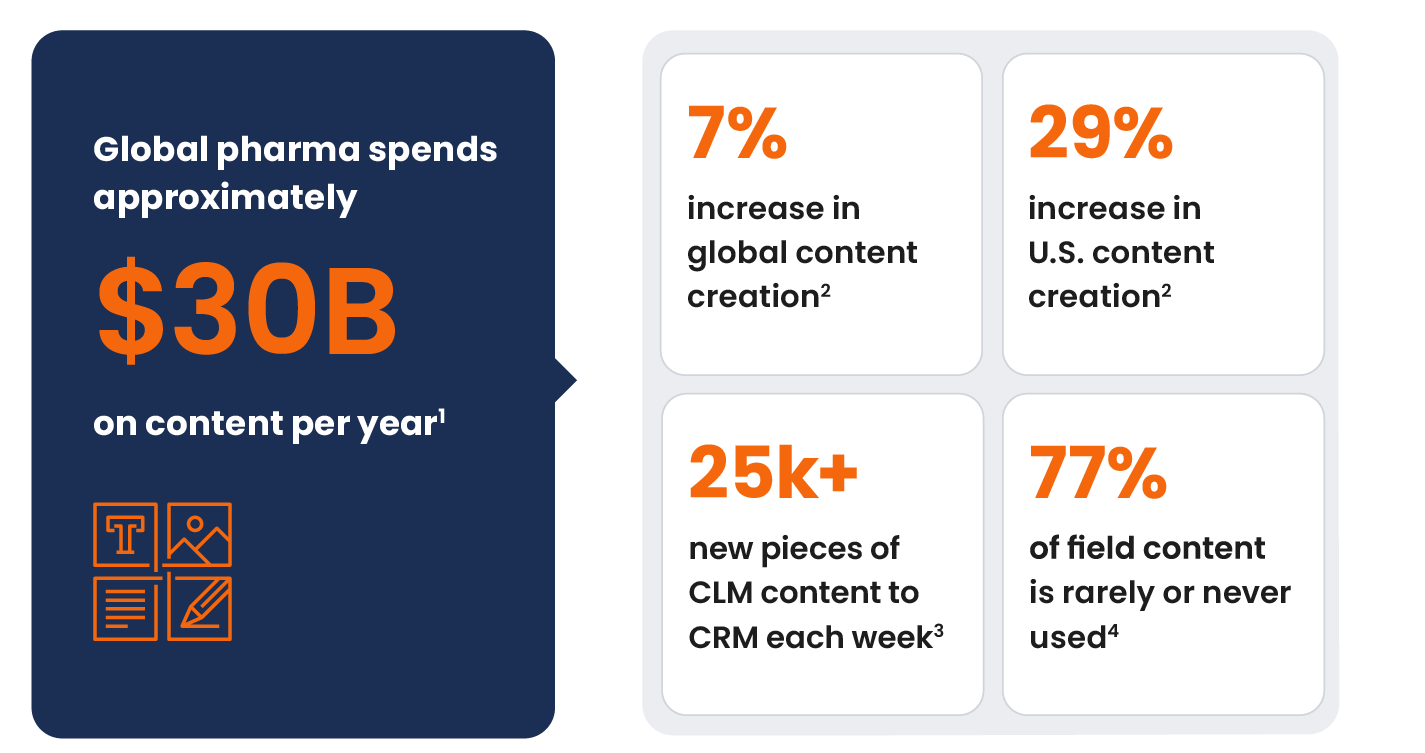
Biopharmas are creating record-high volumes of material to engage healthcare professionals (HCPs) in relevant ways across channels: Production rose 7% globally and 29% in the U.S. in 2023 over the prior year — and the pace isn’t slowing (Figure 1). Meanwhile, field teams rarely or never use 77% of approved content. Participants across the content supply chain are challenged to develop only those assets worth the cost while driving meaningful customer experiences.
1 Healthgrades 2024 Outlook: Pharma Marketing Trends to Watch
2 Veeva Content Benchmark data for U.S., 2023
3 Veeva Vault PromoMats
4 Veeva Pulse Content Metrics, 2025
Content teams rely on highly skilled medical, legal, and regulatory (MLR) experts to create relevant and compliant material. However, they tap into reviewers’ expertise late in the cycle. This process, coupled with stagnant or reduced reviewer headcounts, strains capacity. Without a more proactive role or visibility into content creation, reviewers are perceived as a bottleneck, working through redundant reviews as content volume rises.
“If you want your content to get out quickly and efficiently, companies need to view MLR as a business issue and be invested in trying to solve that,” says Dr. Samin Saeed, vice president of Early Assets Medical at GSK.
More and faster product launches and evolving HCP content preferences create the need to revolutionize the MLR role. AI promises to help accelerate content, but it is one lever. Having aided in improving quality and reducing the workload for MLR teams, its value is now crystallizing as part of a larger opportunity to reimagine end-to-end content operations.
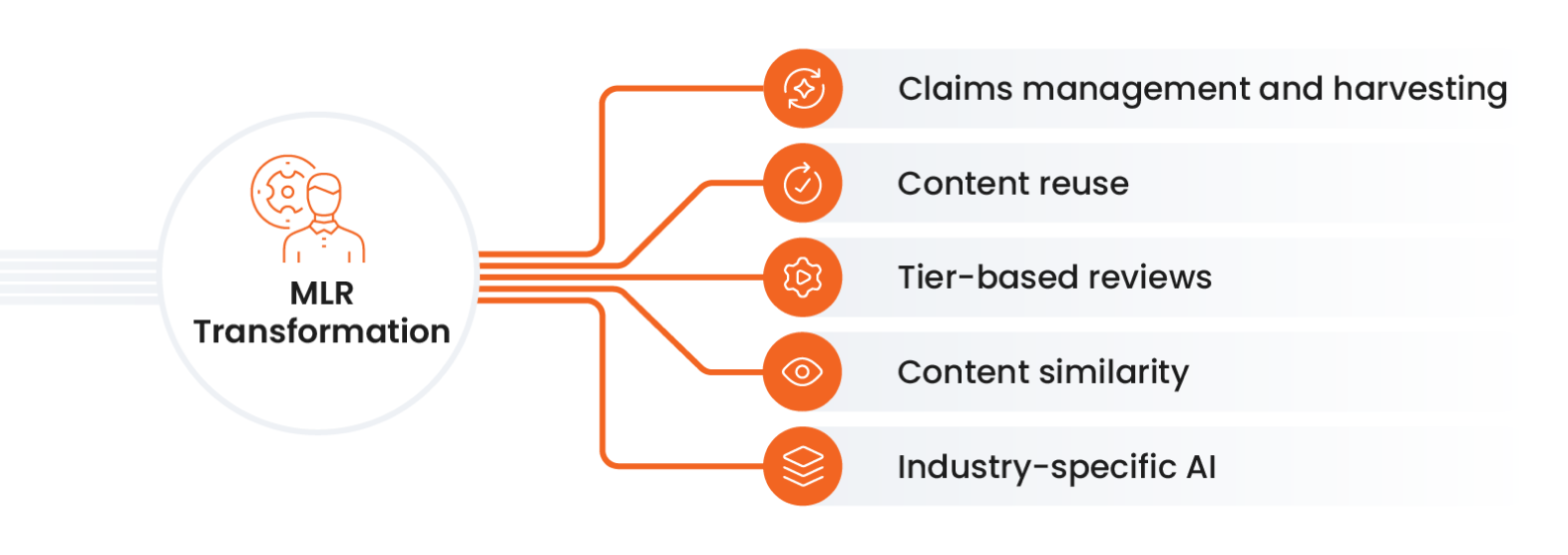
Along with AI, the process innovations supporting transformation in the MLR role include:
- Claims management and harvesting: Digital tools auto-link approved claims to references in a centralized claims library, removing 90% of the work versus manual. Claims harvesting creates claims from link annotations on steady-state documents, making library maintenance easier.
- Content reuse: Once approved, content modules are ready for use in various assets and multiple channels, creating more personalized customer experiences.
- Tier-based reviews: Business rules move a large percentage of content through streamlined tiers compliantly and efficiently, freeing resources to focus on high-value, high-risk material.
- Content similarity: Comparing derivative content with scoring helps reviewers prioritize where to spend time during review and approval.
These advances — coupled with AI products capable of evolving with a company’s unique roadmap — deliver the best return on investment by speeding the full content creation, review, and approval cycle to help transform the MLR role.
"All of the critical pillars that own pieces of the content workflow and lifecycle must be aligned. However, roles needn’t remain static, especially as AI takes effect." Content strategy director at a top biopharma
The value of transforming the MLR role
Because MLR teams receive content in the last mile of production, they communicate back and forth with marketing operations, brand teams, agency partners, and other stakeholders. Rising content volume hasn’t affected MLR review cycle times: The global average for content approval is consistent. But, pre-review processes add time — ranging from five to 150 days for each asset. Reinforcing AI and automation with process improvement, especially during MLR review, is the best way to meet HCPs’ growing content needs.
“All of the critical pillars that own pieces of the content workflow and lifecycle must be aligned. However, roles needn’t remain static, especially as AI takes effect,” says the director of content strategy at a top global biopharma. Bringing reviewers, brand teams and/or agency partners, and marketing operations together early in the content lifecycle is a significant shift. It empowers teams to co-create higher-quality content initially and reduce rework later.
Collaborative, tech-supported teams can produce relevant content faster despite new challenges, such as expedited launches for more indications. New approaches and technology enhance the experience for reviewers and all who touch medical and marketing content.
“It’s critical to focus our resources to manage the MLR review process efficiently for all stakeholders while ensuring we support the business in all our initiatives compliantly,” says Jamie Moccia, manager of MRC and medical operations at Argenx.
"Ensuring compliance with FDA requirements remains a key responsibility of our MLR teams, which AI alone cannot handle.”
AI and the evolution of MLR teams
For years, commercial teams relied on manual, paper-based systems that were slow and error-prone. In response, content teams shifted to digital technologies to streamline workflows, improve collaboration, and spark new ways to develop promotional material. The industry entered a digital renaissance when organizations learned to use digital content investments effectively.
The next transformation is underway as companies test AI to improve efficiency, speed, quality control, and reviewer satisfaction. More than 80% of large life sciences companies are exploring its use for content creation and review processes, tagging, and quality checks, according to Veeva data.
Early reports indicate an opportunity for life sciences companies to derive $5-7 billion in value from AI use, with 25-35% of that total coming from commercial use alone. There’s no one-size-fits-all solution, and the results of AI use in MLR review are still taking shape. But markers of success are emerging (Figure 2).

Industry confidence in AI’s ability to help transform MLR review is growing, with the caveat that reviewers must maintain the final say. Andrea Ward, executive director of global ethics and compliance at Astellas, says that compliance with the FDA and other regulatory bodies mandates human intervention, especially for processes like audit trails and documented proof of compliance: “Ensuring compliance with FDA requirements remains a key responsibility of our MLR teams, which AI alone cannot handle.”
Sabine Post, global content operations lead for go-to-market capabilities at Sanofi, agrees. "While automation aids in approaches like tier-based reviews, human oversight is crucial to maintain accuracy and compliance.”
Marketers’ skill sets and comfort with AI tools outside review — for content creation and hygiene, for example — are still growing. In addition, biopharmas haven’t experienced scalability in using AI for these purposes, but this will change rapidly.
"In our day-to-day, we are not seeing the impact we were promised GenAI would have in the industry. The area where GenAI has the most potential is MLR and it's exciting to see Veeva embrace that with Veeva AI for PromoMats."
The future of reviews
Adopting AI capable of delivering pre-reviewed content that’s ‘90% of the way there’ is a proactive step in reimagining the content supply chain. A new AI application for Veeva PromoMats will soon be available in response to this need. Veeva AI for PromoMats prioritizes quality, speed, and trust by focusing first on MLR pre-review efficiency. Future applications will empower marketers to be greater contributors to a sustainable content lifecycle.
Veeva AI for PromoMats uses a customer-hosted or Veeva-supplied large language model and embeds AI in the content lifecycle. Veeva AI Chat provides a whole new way to use Veeva PromoMats with agentic AI in a context-aware, conversational interface. Quick Check Agent performs quality checks before MLR review and approval, checking for adherence to the following:
- Editorial standards: Quickly identifies spelling and grammar errors, as well as forbidden phrases
- Brand guidelines: Confirms copyright, trademarks, and correct use of privacy statements and images
- Market guidelines: Automates black box warnings, inverted triangles, and ISI or PI inclusion to ensure compliance with changing local health authority regulations
- Channel rules: Checks content alignment with requirements across channels, including unsubscribe options, QR codes, sizing, and accessibility
Veeva AI for PromoMats: Part of a larger AI initiative
Veeva’s AI Partner Program enables customers and partners to build AI applications that integrate with Veeva Vault applications. In addition, a direct data API provides high-speed access to the data needed for AI.
Enhancing review with Veeva AI for PromoMats is a competitive advantage. Reviewers gain time to take a more proactive role in creating content and reducing rework at the end of the cycle. AI-supported MLR teams could deliver:
- Faster reviews by reducing cycle times by up to 75%, according to Veeva estimates
- Improved quality and compliance by focusing reviewer attention on high-risk content
- Enhanced reviewer experience by reducing repetitive, administrative, and manual tasks
- A reduced need for in-person meetings today and their elimination long term
Innovating across the content lifecycle
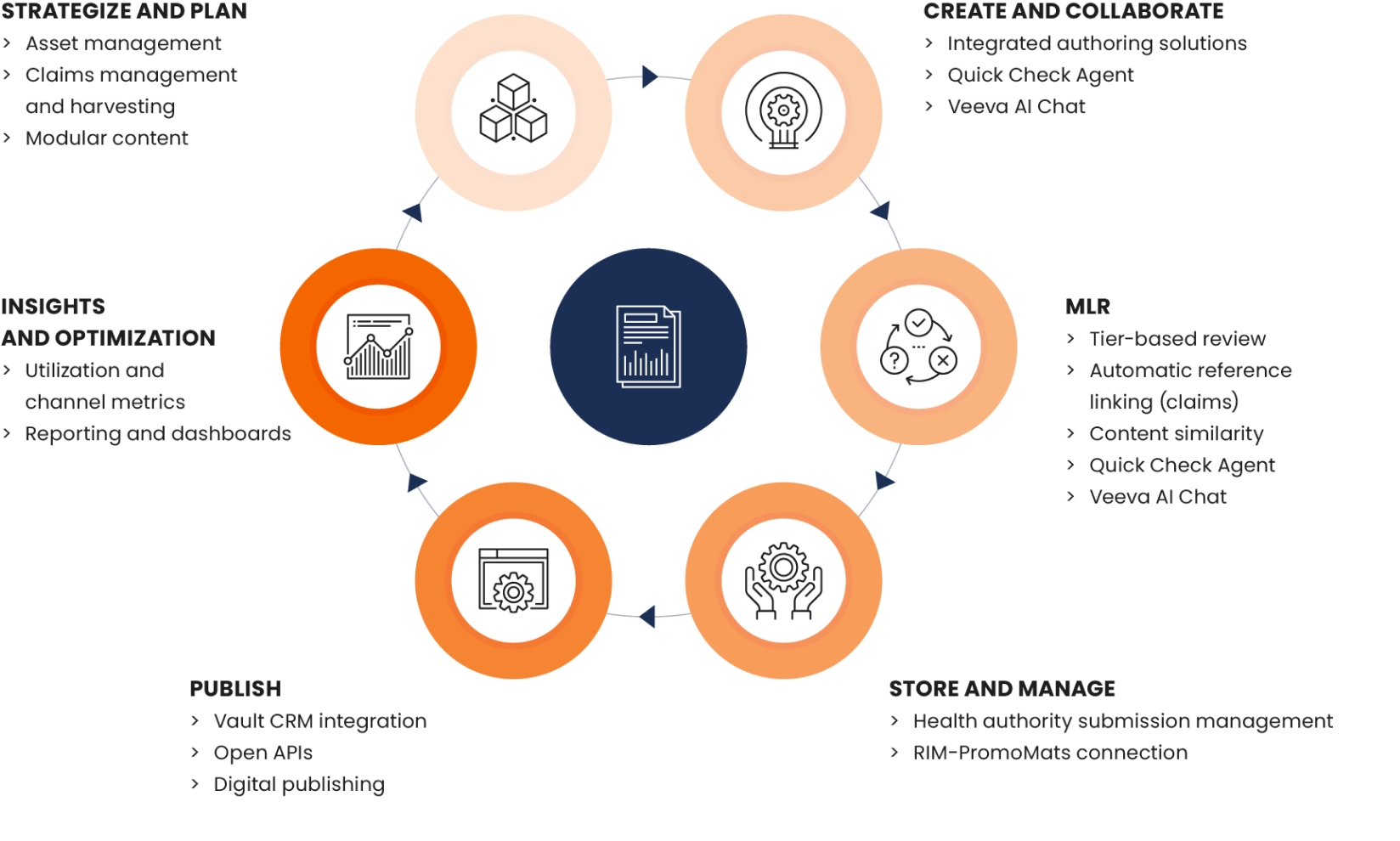
PromoMats is invested in all aspects of content. Product and feature enhancements such as content similarity and claims harvesting are deeply focused on MLR optimization. Veeva AI for PromoMats will provide full support for biopharmas — from strategy to efficient review and post-publication insights on field usage (Figure 3). Reviewers, marketers, brand teams/agency partners, and marketing operations teams collaborate to create faster, more compliant, personalized content for HCPs as volume grows.
"We’ve been talking about AI as an enabler. Finding the balance of automation and human touch allows us to create personalized relationships."
6 takeaways to create efficiencies with AI
When asked how life sciences field reps add the most value, 62% of HCPs said it’s by understanding their needs and sharing only relevant content to make interactions insightful. “We’ve been talking about AI as an enabler. Finding the balance of automation and human touch allows us to create personalized relationships,” says Richard Palizzolo, executive director and head of customer experience at Sobi.
Driving meaningful customer experiences requires thoughtful adoption of AI, automation, and process improvements. Keep the following guidelines in mind to safeguard investments in content efficiencies:
- Select a committed partner: Reliable AI providers will dedicate the upfront investment needed to innovate and the resources for long-term maintenance. Partners should show a roadmap of their AI plans and provide avenues for feedback.
- Prioritize scalability: Avoid custom AI tools not designed to work within content platforms or require complex integrations with business intelligence tools. Consider essential groundwork, such as standard taxonomy, critical for deploying AI at scale.
- Make AI one part of the plan: Focus resources on strategic priorities like insights and operational excellence parallel to AI. A lack of caution about AI investments and integration can drain limited resources and distract from key business objectives.
- Define standard operating procedures: Document and communicate guidelines for stakeholders and users before incorporating AI products. Put people first by building a change management culture that addresses knowledge and skills gaps.
- Stay focused on a single impact area: Carefully select initiatives to get the best ROI from AI use. AI for MLR pre-review, which prioritizes content quality over quantity, will deliver value faster.
- Embrace progress over perfection: Establish a baseline for comparison before implementing AI. Tracking before and after time in pre-review helps build alignment and support for AI follow-on initiatives.
A wait-and-see approach to harnessing AI products' potential in accelerating compliant content equates to lost ground. Content teams need the tools and accompanying process improvements to help drive better patient outcomes and business success today.
Improve reviewers’ experience and prepare content teams to create faster, more compliant, personalized content as volume grows.
