Biomapas Takes the Lead in Closer
Service Provider and Sponsor
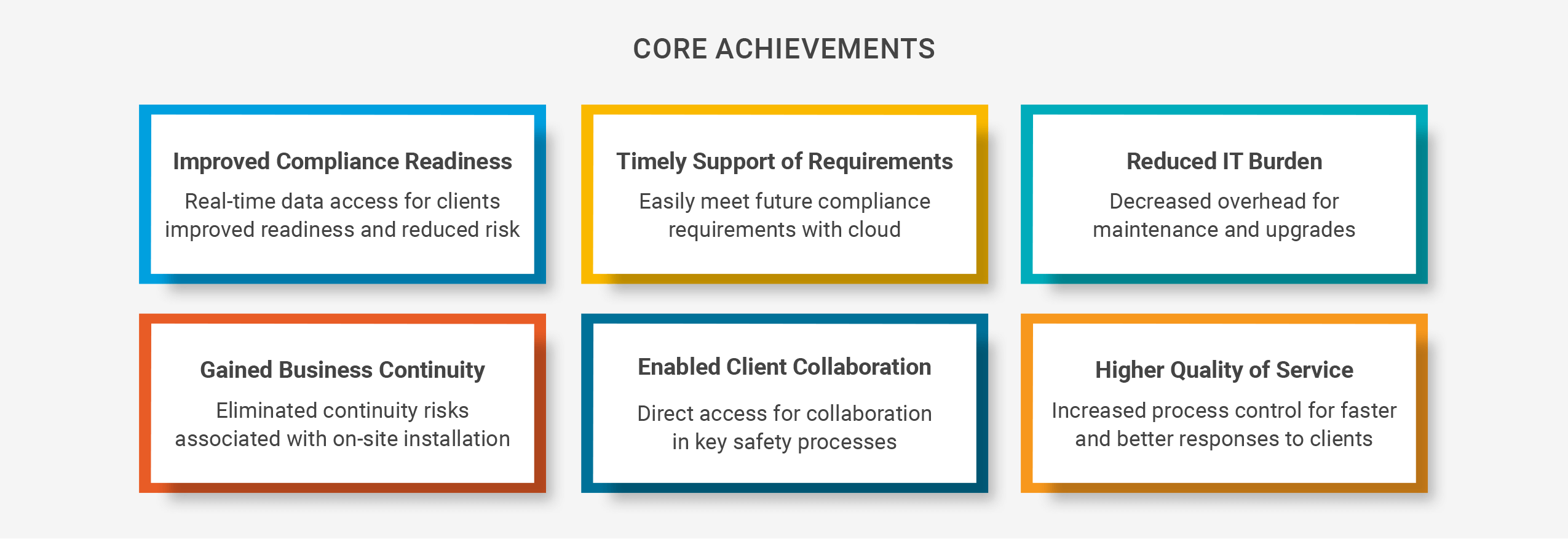
Drug Safety Collaboration
"Even though we manage the data ourselves, with Veeva Safety we can offer customers access to as much of their data as they wish – in real time. This allows them to participate directly in workflows that they want to oversee."
Over the past few years, pharmacovigilance caseloads have been rising, with a growing number of reported adverse events. At the same time, more global regulations with diverse requirements have increased demands on pharmacovigilance teams.
These pressures are driving more companies to outsource pharmacovigilance functions to specialized CROs such as Biomapas, a Europe-based company that offers safety, clinical research, regulatory, and medical information services with a focus on Europe (EU), Eurasia (EAEU), Middle Eastern/North African (MENA), and Latin American (LATAM) markets. The company offers global and local pharmacovigilance (PV) services, including the role of EU / EAEU / UK qualified persons responsible for pharmacovigilance (QPPVs) and comprehensive activities for a PV system, such as managing individual case safety reports (ICSR), pharmacovigilance system master files (PSMF), aggregate safety reports, and global and local literature screening. As a global company, Biomapas helps clients meet differing regional requirements, adapt to legislation changes, submit reports, and manage documentation in different languages.
Unifying data with greater accessibility
Until 2021, Biomapas relied on an outdated on-site database and point solutions to manage safety information. The result was fragmented, disconnected data. System limitations made it difficult to manage data from different sources and to find and report on required information for inspections and regulatory compliance.
The company searched for a safety solution that would facilitate compliance and reduce IT maintenance requirements. Biomapas ultimately selected Veeva Safety, an easy-to-use, easy-to-configure cloud solution that stays up to date with new regulatory requirements. With robust data segregation, Biomapas can now support multiple clients on the same system while ensuring each client’s data is only accessible to authorized users. A flexible security model also provides different levels of access, allowing users to see specific data or reports, or perform key tasks.
Moving to a modern safety solution
Vault Safety has removed many of the limitations of the company’s former safety solution. “We now have better control of and access to the data and can offer our customers a higher level of service,” says Chief Operating Officer Pharmacovigilance (PV) / Regulatory Affairs (RA) / Medical Information (MI) Martijn van de Leur.
Being able to trend data and run reports independently without the need for vendor support has made operations more flexible, says van de Leur. With past systems, the company had to pay vendors to pull data and create custom reports. “I felt that our data was being held hostage,” he says. Now, Biomapas and its clients can easily view, customize, and create reports.
Leveraging a multitenant Veeva Safety cloud solution, Biomapas can more efficiently support clients. “We don’t need 10 or more different logins for the different client environments as we did in the past,” says QPPV Office Manager / Head of PV Technologies Gediminas Zvicevicius. “The model is set up so that every object and every document has an organization assigned to it, and it is easy to add organizational users.”
Better control and more flexibility
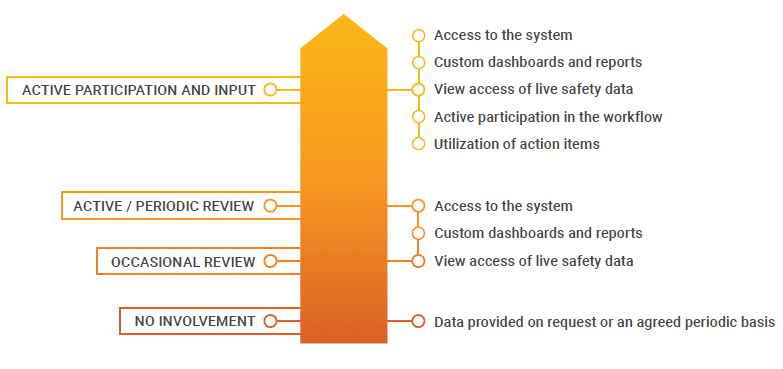
Biomapas can now collaborate more effectively with clients by offering biopharmas the flexibility to define the level of involvement in safety processes. Some customers, particularly emerging biotechs, may only want to view the data or have oversight of operations, while larger companies often appreciate the ability to work more closely with the CRO. “Other databases don’t allow customers to take an active part in the workflow,” says van de Leur. Veeva Safety’s capabilities allow customers to be involved in medical review and easily demonstrate compliance, both of which can improve overall efficiency and quality management.
Drug safety teams at Biomapas find the modern safety solution to be intuitive and user friendly, says Zvicevicius. “The Veeva Safety menus are well organized, and the layout is clear,” he adds. In addition, it helps users manage action items and queries related to each specific case, and to check email communications.
As a cloud solution, Veeva Safety has considerably reduced Biomapas’ overhead. “We don’t have to maintain an in-house system, our own IT servers, or worry about network security,” says van de Leur. “We gain administration independence and can configure the application based on current and future needs. Other systems that the company had evaluated did not offer this capability, van de Leur says.
LEVELS OF CONTROL IN VEEVA SAFETY
Scalable, secure, and validated in the cloud
“Five years ago, the industry asked whether it was even acceptable to have a safety database in the cloud. Now, the question is, ‘why wouldn’t you have one there?’” van de Leur says. The notion that in-house solutions are more secure than cloud-based systems is wrong, he adds. “I wouldn’t want a few IT staffers to be responsible for such a sensitive and important database. I trust a larger company that has teams dedicated to security.”
Cloud-based solutions are scalable and accessible from anywhere. “The performance of our on-premise database slowed as the amount of data and number of users increased. With Veeva Safety, performance is always consistent, no matter how many users we have or how much information is loaded into the system,” says van de Leur.
The implementation was well-planned and executed smoothly with Veeva services and Biomapas’ safety team. “From the beginning, the project had a clear roadmap. It was ambitious and, in the end, all key objectives and timelines were met,” says van de Leur. “Every time we needed something, it was there.”
With three pre-validated releases a year that provide new capabilities, Biomapas now has a solution that grows with the business and keeps up with changing regulations for easier compliance.
Advancing safety with automation
For the life sciences industry, helping ensure patient safety by reducing tedious manual processes in case processing and other areas of pharmacovigilance is key. There is an urgent need for technology to automate more of the work. This will likely proceed in stages, starting with the most basic aspects of case processing, such as taking a form and converting it into structured data and populating relevant fields in the safety database.
Biomapas took a first step toward a more automated future by modernizing safety management with a solution that is more than a database. Now, data that had once been disconnected is available in one place and accessible in real time to all parties. And the business can seamlessly scale with more data and new regulations.
The ability to tailor each client’s direct access to data and their level of participation in safety processes based on business needs provides a distinct competitive advantage. As global regulations become more stringent, Biomapas’ enhanced flexibility and control will help reduce risks for its pharma clients as well as the patients who depend on its therapies.



