Simplify and Standardize
Clinical Trial Execution
Unify clinical systems and processes for efficient, high-quality studies
Fortrea eliminates siloes and 90% manual effort with a unified platform
Veeva Clinical Operations
Veeva Clinical Operations unifies clinical systems and processes on a single cloud platform to enable end-to-end trial management.
Clinical Operations applications share a common data model, which allows for the consolidation of clinical operations applications in one Vault.
Veeva eTMF
The leading trial master file application used to ensure the quality, timeliness, and completeness of a TMF.
Veeva CTMS
An enterprise trial management system that provides end-to-end study management and monitoring capabilities.
Veeva Payments
Manages payments to research sites and tracks study budgets.
Veeva Study Startup
Manages the start-up activities of a trial, including feasibility, qualification, and activation of research sites.
Veeva RTSM
Used by sponsors, CROs, and sites on clinical trials to randomize patients and manage trial supplies.
Veeva Site Connect
Provides one application for sites and sponsors to work together. It simplifies the flow of information during start-up, execution, and closeout.
Veeva Study Training
Manages GCP and study-specific training for research sites, CROs, and sponsor personnel.
Veeva Disclosures
Manages the sharing of study registrations and results disclosures with public registries.
Veeva OpenData Clinical
Provides accurate, compliant data about global investigators and research sites.
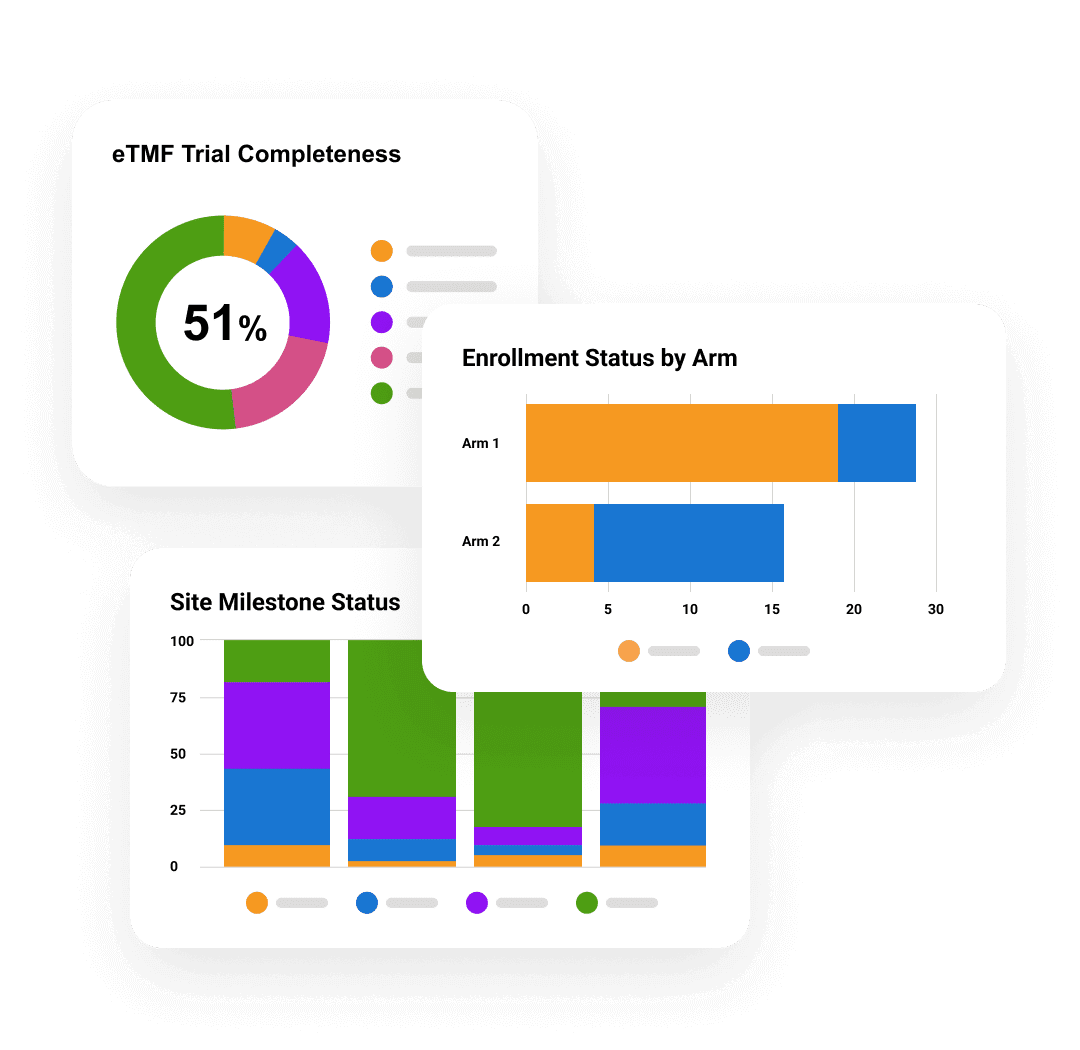
Veeva AI for Clinical Operations
See in Action
TMF Intake Agent
Automatically classifies, indexes, and adds key metadata to incoming TMF documents, reducing manual work and improving TMF quality.
Quality Check Agent
Reviews a single or set of documents for completeness and accuracy, improving content quality and supporting TMF readiness.
Veeva Connections
Veeva Connections are Veeva-delivered integrations that seamlessly transfer data and documents between Vaults. Veeva Clinical Operations is connected with Veeva Clinical Data, Veeva RIM, and Veeva Safety to streamline cross-functional processes.
Impact
Improve Trial Performance
Streamline clinical trials from study start-up to closeout
40%
cut in TMF reconciliation time with Veeva eTMF
75%
reduction in SDV preparation time per trial
45%
faster to enroll the first subject after site initiation
Customer Success
Trusted by Sponsors and Contract Research Organizations
More than 500 companies accelerate trials with Veeva Clinical Operations.












Watch Video
Jazz Pharmaceuticals Supports its Hybrid Operating Model with Veeva eTMF and Veeva CTMS

Read Article
Inhibrx Brings all GxP Systems In-House to Build a More Efficient Operating Model

Watch Video
Boehringer Ingelheim Improves Patient Outcomes with a Connected Clinical Environment

Read Article
CSL Behring and Takeda Advance Clinical Development

Watch Video
ImmunityBio Runs More Efficient Trials with a Lean Team

Watch Video
Fortrea Unifies Clinical Operations to Improve Efficiency

Watch Video
AstraZeneca Drives Transparency with Veeva Clinical Operations