Veeva SafetyDocs
Regulated Safety Content and Process Management
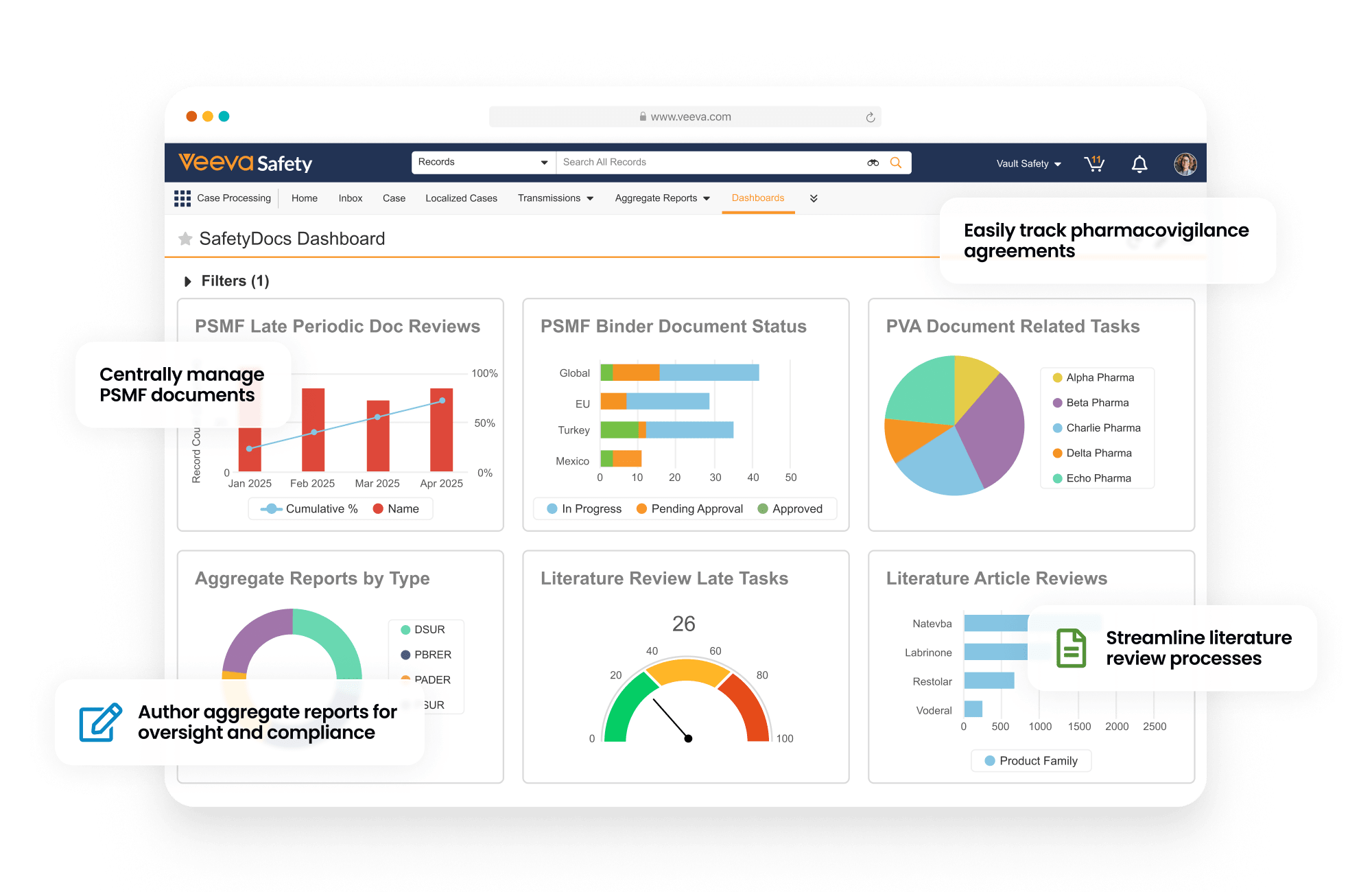
SafetyDocs manages pharmacovigilance-related content and processes. It provides solutions for the management of pharmacovigilance system master files (PSMF), pharmacovigilance agreements (PVAs), risk management plans (RMPs) and additional risk minimization measures (aRMMs), aggregate reports, and safety signal investigations.
Learn more about how Veeva SafetyDocs enables more efficient safety content and process management.
Announced 2019 Status Mature Customers 11-50
Overview
Regulated Safety Content and Process Management
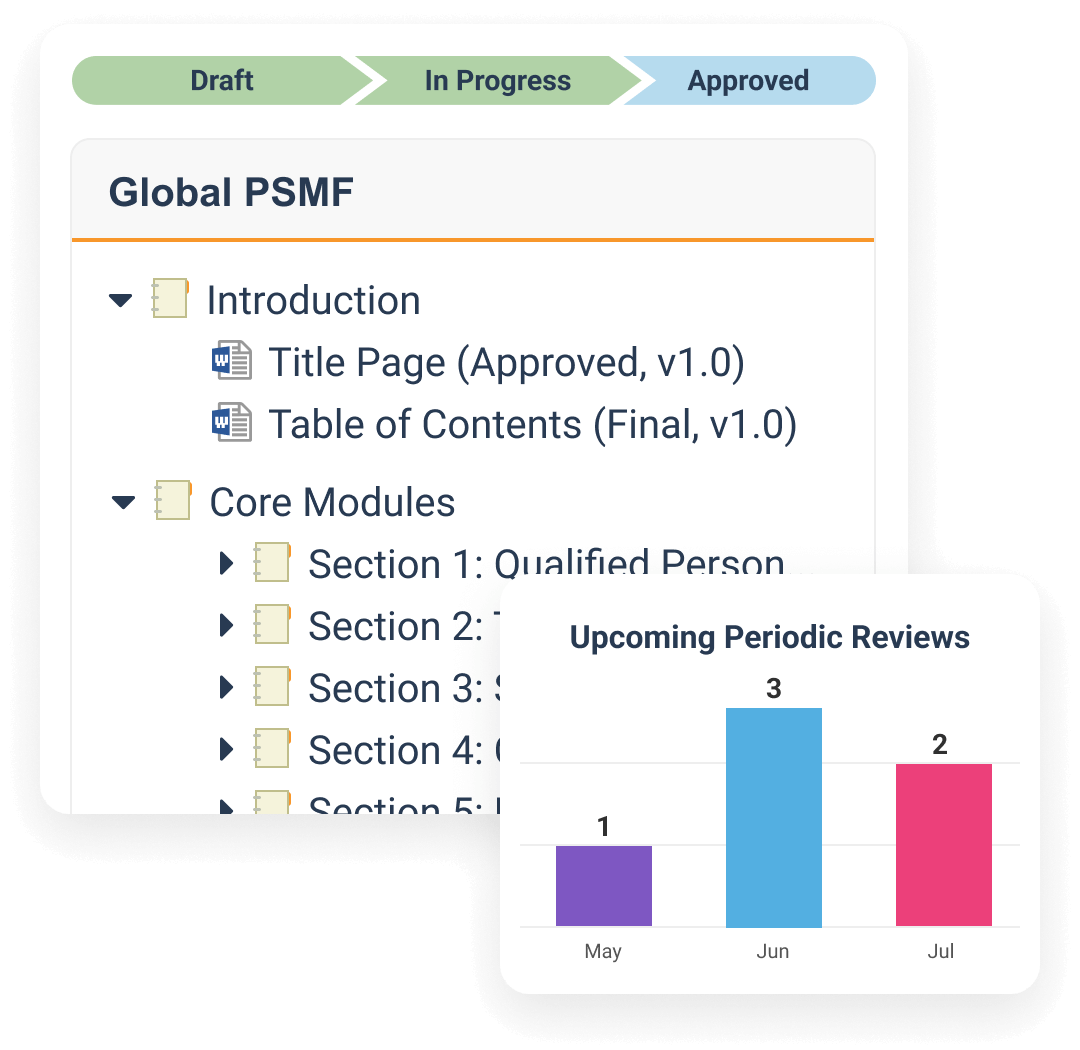
SafetyDocs manages pharmacovigilance-related content and processes. It provides solutions for the management of pharmacovigilance system master files (PSMF), pharmacovigilance agreements (PVAs), risk management plans (RMPs) and additional risk minimization measures (aRMMs), aggregate reports, and safety signal investigations.
Learn more about how Veeva SafetyDocs enables more efficient safety content and process management.
Why Veeva SafetyDocs
Greater operational efficiency and compliance
Customer Success
Manage safety-related processes and content in a validated solution designed for pharmacovigilance




Resources
Explore and Learn
Read Features Brief
Veeva SafetyDocs Features Brief
Read Blog
Holistic Risk Management in Safety: From Global Plans to Local Action

Read Features Brief
Veeva SafetyDocs PVA Management Solution Brief

Read Blog
Connected Systems: Fastest Path to an Inspection-Ready PSMF
Learn More
Centrally Manage Pharmacovigilance Processes and Content

Read White Paper
Globalize PSMF Management for Greater Efficiency