Veeva RTSM
Enterprise Standard RTSM
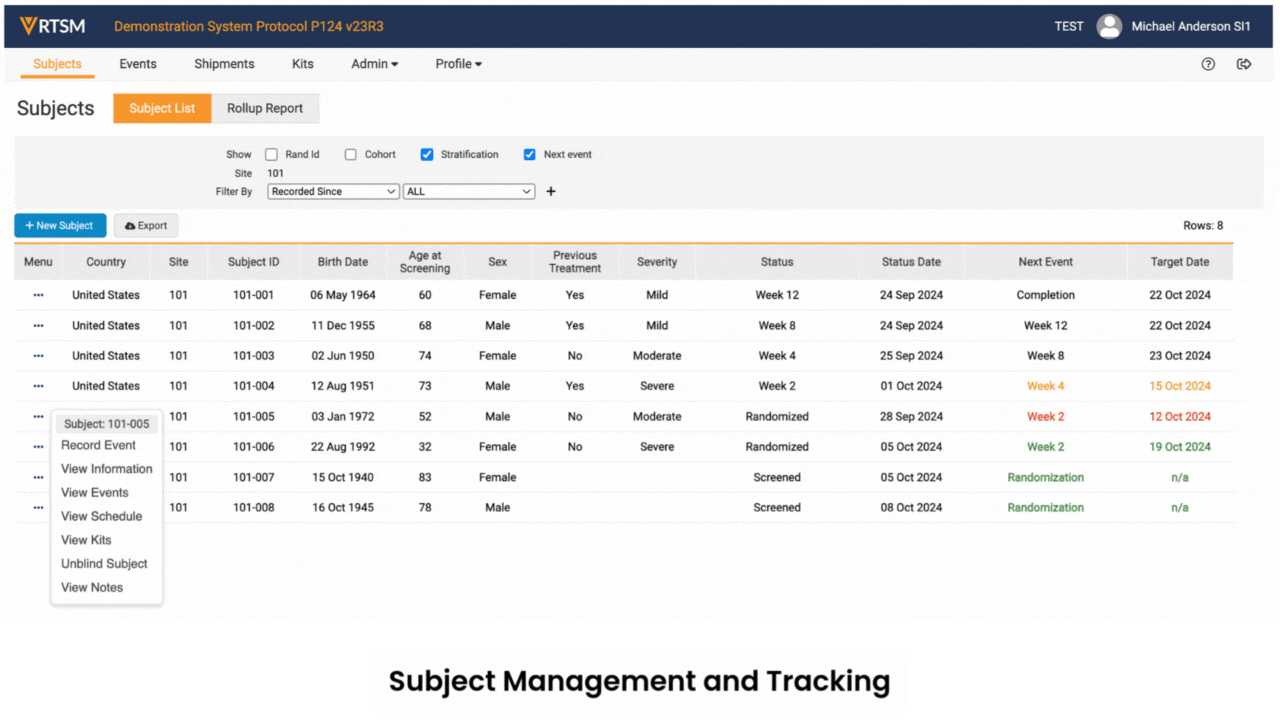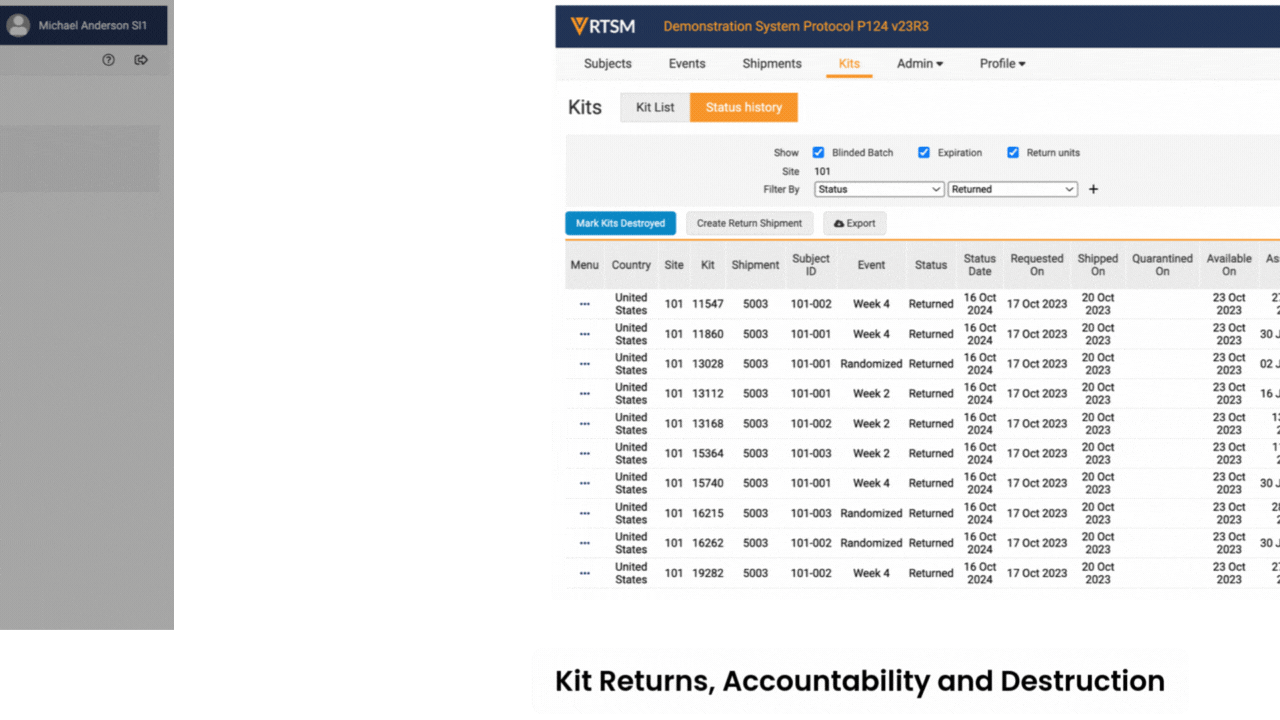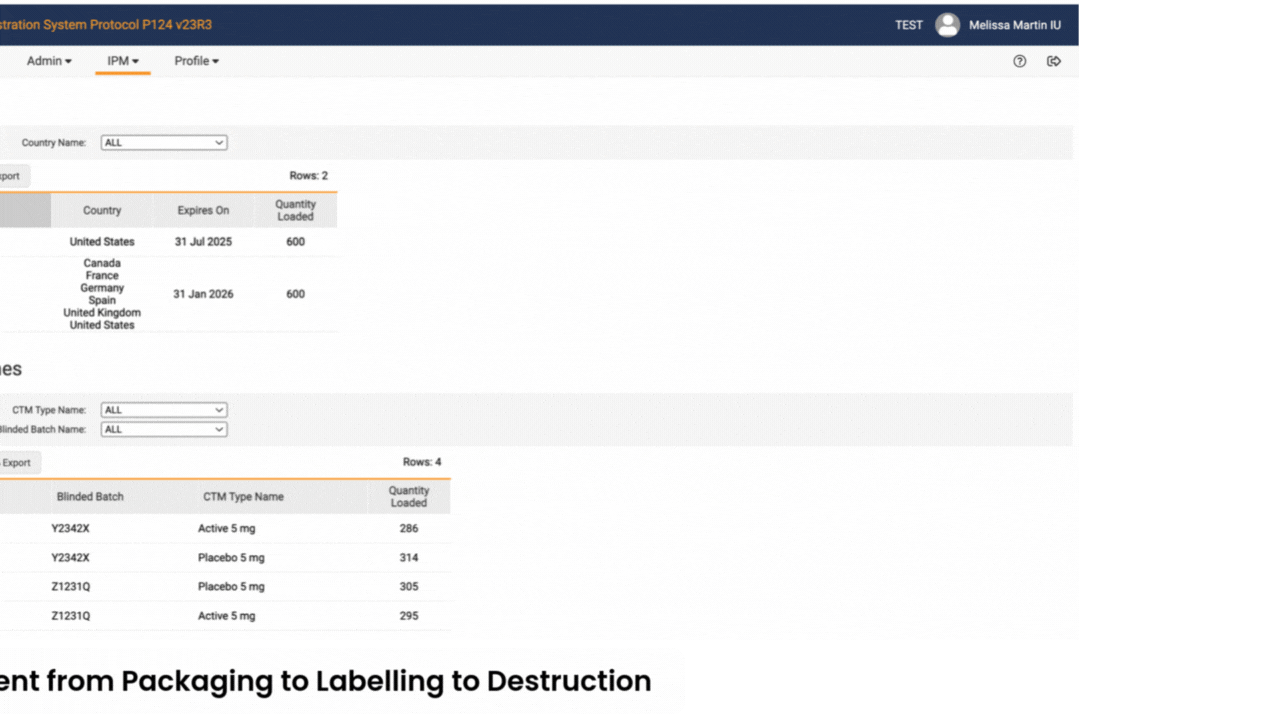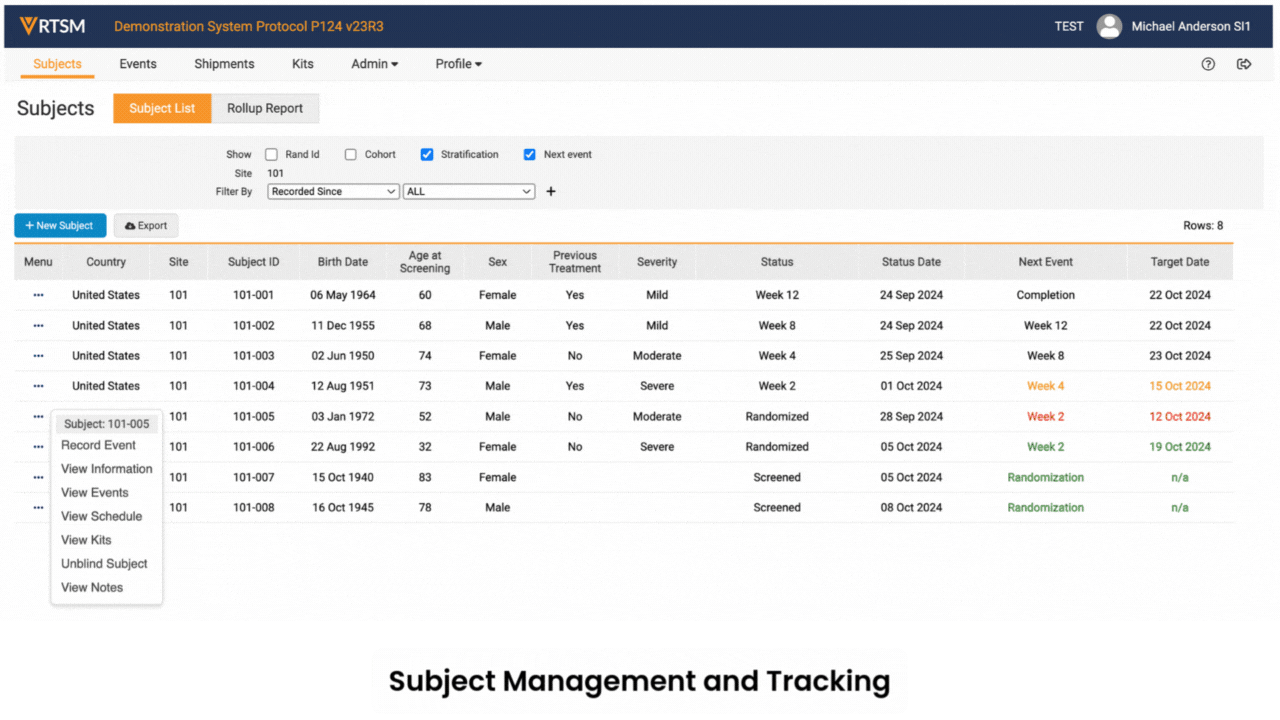
RTSM is used by sponsors, CROs, and sites on clinical trials to randomize subjects and manage trial supplies.
Sites log in to Veeva RTSM to record screening, randomize subjects, get kit assignments, and perform emergency unblinds (as needed). RTSM ensures that sites have all the supplies needed at the right time through either basic or predictive supply algorithms. Productized connections transfer patient data to Veeva EDC and screening, randomization, and visit data to Veeva CDB.
Veeva RTSM is implemented, managed, and fully supported by Veeva.
Announced 2010 Status Mature Customers 51-100
Register today for our exclusive RTSM event at the 2026 European R&D and Quality Summit
Overview
Enterprise Standard RTSM
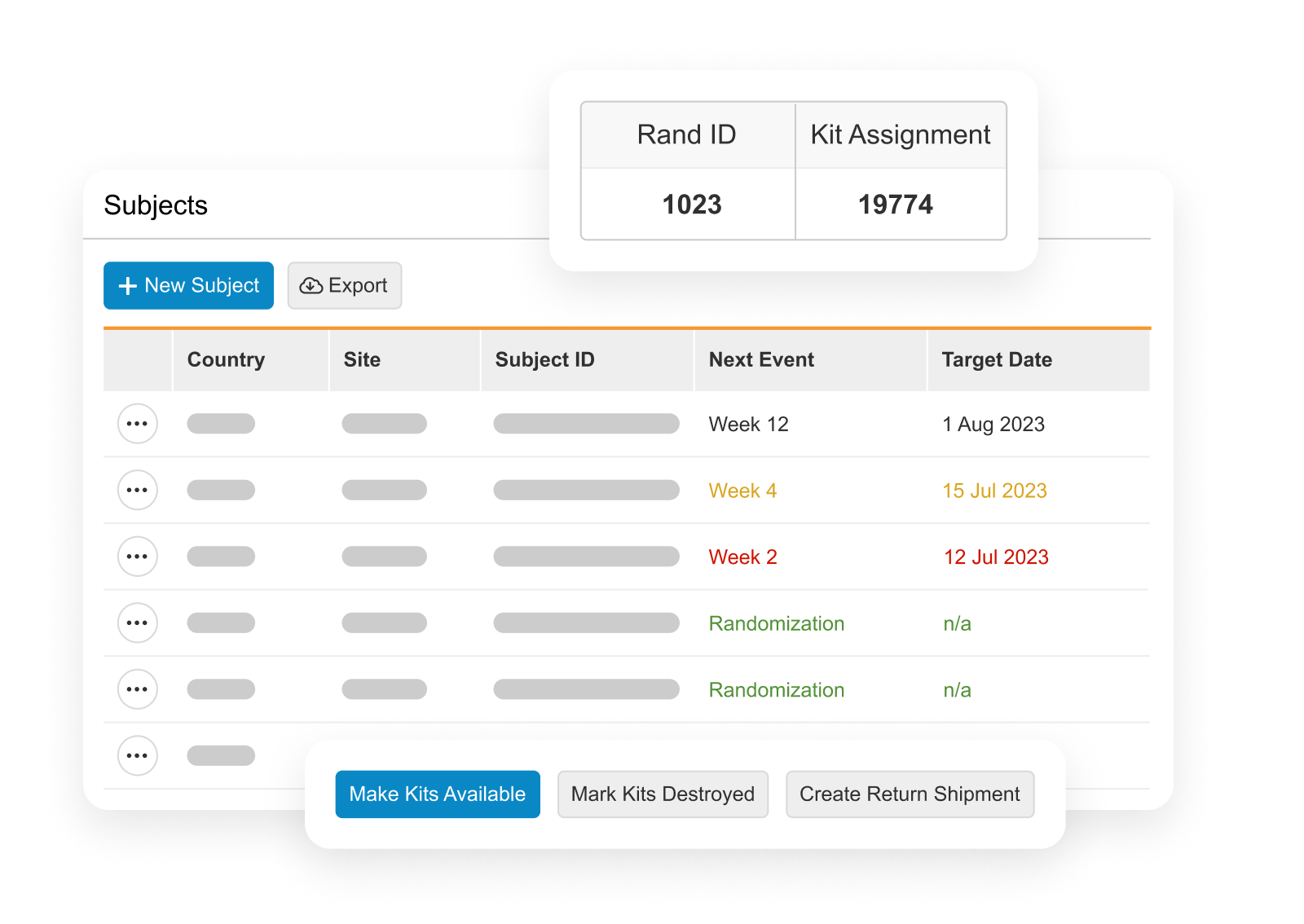
RTSM is used by sponsors, CROs, and sites on clinical trials to randomize subjects and manage trial supplies.
Sites log in to Veeva RTSM to record screening, randomize subjects, get kit assignments, and perform emergency unblinds (as needed). RTSM ensures that sites have all the supplies needed at the right time through either basic or predictive supply algorithms. Productized connections transfer patient data to Veeva EDC and screening, randomization, and visit data to Veeva CDB.
Veeva RTSM is implemented, managed, and fully supported by Veeva.
Why Veeva RTSM
Seamless trial execution and site experience
Resources
Explore and Learn
Read Features Brief
Veeva's Enterprise Standard RTSM Solution

Read Features Brief
Creating Cohorts and Dose Levels in Veeva RTSM

Watch Video
How Julius Clinical Elevates Their Clinical Trials with Veeva RTSM

Watch Demo
Veeva RTSM to Veeva EDC Integration
Watch Demo
Veeva RTSM to Veeva CDB Integration

Read Services Brief
Dedicated Biostatistics Services with Veeva RTSM

Read Features Brief
The Fundamentals of Resupply in Veeva RTSM

Read Features Brief
Optimize Drug Supply Outcomes with Dose Probabilities Functionality

View All Resources
Full Veeva RTSM Resource Hub

