Features Brief
Veeva Product Surveillance Features Brief
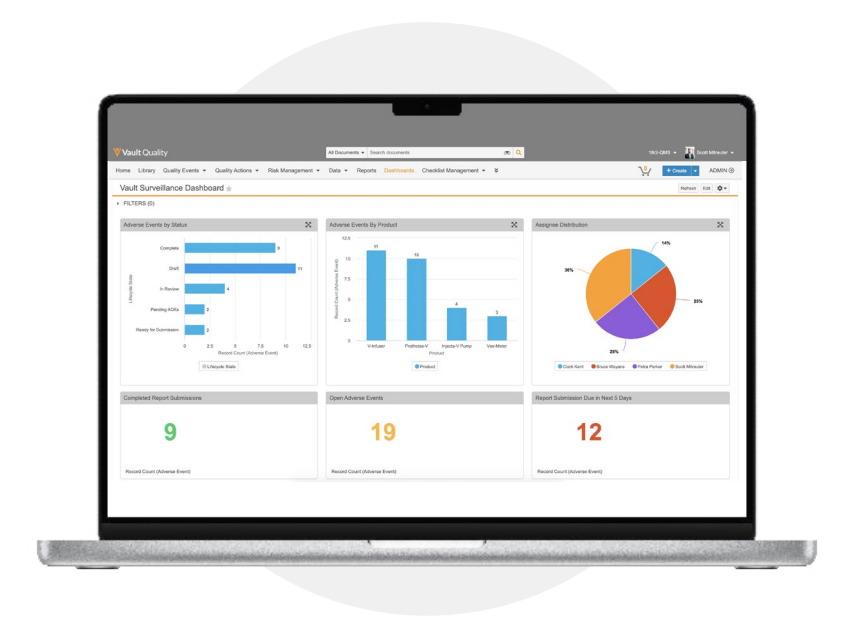
Simplify and standardize global postmarket surveillance
Veeva Product Surveillance simplifies and standardizes postmarket surveillance for medical devices, improving product safety, reliability, and quality. Fully automated electronic health authority submissions and non-electronic submission outputs ensure timely adverse event reporting. Seamless connection with quality and regulatory processes enables proactive complaints handling, accelerating continuous innovation.

Business Benefits
Improve product quality and patient safety.Proactively identify and resolve product quality issues for greater reliability, safety, and compliance.
Ensure submission timeliness.Meet submission timelines with an intelligent, global reportability decision tree with country-specific criteria.
Real-time visibility and end-to-end control.Make informed business decisions with real-time visibility into submissions and complaint-handling metrics.
Features
Global Reportability Decision TreeStandardize and consolidate the complaint reportability process for various health authorities through a global decision tree.
Reporting Timeline ManagementEfficiently manage event-specific reporting timelines to ensure compliance and timeliness across various health authorities. Enable quality and regulatory teams to allocate resources and prioritize submissions effectively.
Automated Adverse Event ReportingBuilt-in XML payload generation and electronic data interchange (EDI) gateway provide a fully automated electronic submission for the FDA electronic medical device reporting (eMDR). Additionally, supports non-electronic submission for the EU manufacturer incident report (MIR).
Interactive Dashboards and ReportsReal-time, interactive dashboards provide clear visibility into inefficiencies and bottlenecks that cause processing and reporting delays. Take action directly from reports to resolve issues and complete tasks to speed the submission process.
Configurable Event Management WorkflowsAutomate and track events with standard and configurable workflows that provide assignment, routing, email notifications, escalation, and tracking of tasks for groups or individuals.
Part of Veeva Quality CloudSeamless connection to Veeva Quality Cloud enables end-to-end quality management improving product quality and patient safety. Unification with core quality processes, such as CAPA management and content management, eliminates the need to build and maintain complex cross-system integrations.
Veeva Quality Cloud
Veeva Quality Cloud unifies historically disconnected quality systems and processes on a single cloud platform. It enables seamless management of quality events from event origination to changing controlled content and completing training requirements. Managing quality processes (QA and QC), critical documentation, validation, and training in one system simplifies and accelerates event identification, correction, and change management.
The unified applications drive continuous quality improvements while meeting global compliance requirements. Veeva Quality Cloud includes Veeva QMS, Veeva QualityDocs, Veeva Validation Management, Veeva Station Manager, Veeva LIMS, and Veeva Training applications.
