White Paper
A Blueprint for Clinical Trial Transformation: Results from Three Top 20 Biopharmas
Discover how Boehringer Ingelheim, Novo Nordisk, and Bayer have embedded efficiency, innovation, and collaboration to scale sustainable change
A recent machine learning analysis of over 16,000 clinical trials has shown that trial complexity has increased over the last 10 years, across all phases. Often, this is driven by the need to address previously unmet therapeutic targets and address regulatory requirements. More complex trials often result in longer timelines to bring medicines to patients, a higher likelihood of protocol changes, higher patient and investigator burdens, and greater chances of errors and biases. These are a few of many motivations for sponsors to transform their clinical technology and processes, in favor of a simpler trial environment [Figure 1].
This white paper highlights the recent clinical transformations of three top 20 biopharmas — Boehringer Ingelheim, Novo Nordisk, and Bayer — and includes the business cases, operating models, change management tactics, and results.
Turning vision into success: Case examples
A vision for change succeeds when it’s supported by a culture committed to new ways of working. This involves getting everyone aligned on shared goals, measuring progress, and clearly communicating the long-term benefits of the transformation. The following case studies demonstrate how leading organizations have used a clear vision, strategic planning, and dedication to improve efficiency, foster innovation, and enhance collaboration in clinical data management and clinical operations.
BOEHRINGER INGELHEIM
Replaced 40+ systems with a single platform across functions

Boehringer Ingelheim underwent a significant clinical transformation driven by the need to speed up time to submission. The cross-functional teams aimed to accelerate clinical programs by at least 300 days, and create more value from the data generated in its clinical trials.
The company’s vision was to consolidate fragmented processes onto a connected technology platform and empower its workforce. A comprehensive analysis revealed an opportunity to future-proof the system landscape. The biopharma company wanted to adopt a digital-first mindset to realize its platform goals – prompting the need for a foundational change.

Boehringer Ingelheim planned a simultaneous implementation of four Veeva Vaults – Clinical Data, Clinical Operations, Regulatory, and Quality – under its One Medicine Platform initiative. The team initiated the design stage for each Vault in parallel to understand the data model and process dependencies. Based on the initial design phase and the individual requirements of the different areas, the team opted for a phased go-live approach, starting with clinical data management as the team needed to replace a legacy system.
This phased implementation allowed teams to capture crucial learnings from each go-live, applying them to subsequent Vault deployments.

Efficiency: Previously, site setup in the EDC system was manual, disconnected, and time-consuming. Boehringer Ingelheim implemented the Veeva Clinical Operations-EDC Connection to automatically enter site setup from CTMS into EDC. This saved significant time for data transfer, monitoring, and support across dozens of studies. “This is a clear use case for where the platform is providing its value,” comments Martin Heckenberger, capability owner, clinical data collection, Boehringer Ingelheim.
In addition, implementing a modern EDC allowed the team to improve study build efficiency by bringing electronic case report form (eCRF) builds in-house from a functional outsourcing model. By upskilling internal data managers, this reduced handovers and removed the need for edit checks and custom functions, increasing overall efficiency and saving costs.
Innovation: The company transitioned from a complex environment of 40+ systems and 70+ interfaces to a single clinical platform. This consolidation accelerated its pace of innovation, allowing for system updates that previously took years to complete. For recruitment tracking, Boehringer Ingelheim replaced three custom interfaces across four systems with an automated, cross-functional data flow. The Veeva Clinical Operations-EDC Connection eliminated weekly, error prone data transfers and provided “a single source of truth in near real-time,” according to Heckenberger.
Collaboration: Previously, siloed communications burdened sites with multiple contacts from different teams. The clinical transformation improved collaboration with sites by minimizing fragmented processes and through unified communication.
Internal enablement was also an important component: “We wanted to bring all processes to a connected technology platform, and to empower our people. We wanted to make it clear which role has to do what and who is empowered to make which decisions,” explains Heckenberger.
“We wanted to bring all processes to a connected technology platform, and to empower our people. We wanted to make it clear which role has to do what and who is empowered to make which decisions.”
NOVO NORDISK
From implementation to scale-up within 12 months with a dedicated change management team

Novo Nordisk needed to scale for growth and enable innovative trial design elements in new therapy areas. To do this, the company set out to simplify its digital landscape and improve user satisfaction. In partnership with its CRO partner, the company sought to replace its ‘homegrown’ solutions with a unified platform to ensure global consistency, quality, adoption, and accessibility.
“It’s not just about enabling innovative trial design and simplifying the digital landscape. Equally as important is improving end user satisfaction with the systems and increasing our process efficiency.”

Novo Nordisk began with the strategic decision to standardize on a single clinical data platform across all regions and therapeutic areas. The company adopted Veeva EDC and CDB in 2024, with five trials live on the platform within 12 months and a further 20 planned for go-live in the following six months.
The success of Novo Nordisk’s transformation was deeply rooted in its comprehensive change management strategy with its CRO partner. This involved several teams with specific focuses, including dedicated efforts for site and CRA engagement, safety, and clinical adjudication. Staff captured critical end-user experiences, and subject matter experts were actively involved in workshops early in the process.

Efficiency: Early targets indicate reduced study set-up cycle times by 50% in initial trials, a 20% reduction in time and effort for study build, and a 20% reduction in time spent cleaning and monitoring data. The team is also on track to achieve a 1-day EDC build. These projections stem directly from streamlining processes and empowering users through direct system access.
Innovation: With a solution-oriented mindset, Novo Nordisk launched the DataNow program, dedicated to digitizing processes for ‘better, faster, and cheaper trials’. This transformation has paved the way for new, innovative trial designs. For instance, a model study with four indications in one protocol was set up in just two weeks, showcasing the team’s ability to initiate complex trials in the system.
“What’s really exciting me is that once we have the connection between OpenStudyBuilder and Veeva EDC and CDB in place, with a click of a button I can pull out the digital specification from OpenStudyBuilder for my trial, load that data in to Veeva, and then we can automate the trial build. That’s where the magic is going to happen.”
Collaboration: Novo Nordisk focused its change management approach on processes that could significantly reduce administrative burden on people. For example, the reconciliation of serious adverse events (SAEs) between the safety database and EDC was previously a cumbersome process. It involved data managers manually exporting data to spreadsheets, sending it to safety teams for comments, and then re-entering information as queries. By giving its safety team direct access to Veeva CDB, Novo Nordisk eliminated a significant administrative burden for data managers, removed error-prone spreadsheet tracking, and gave safety teams access to live data. This was a “win overall that took a little bit of creative thinking and collaboration,” notes the CRO’s implementation manager.
BAYER
Projected 20% effort savings through connected clinical data flow

Bayer’s move into cell and gene therapies brings new challenges as a large pharma company: working with a network of drug innovation providers and start-up biotech companies means adapting to a faster pace and providing them with the infrastructure to develop new therapies. This move also brings greater scientific complexity and new research site collaborations. Bayer was driven by the vision of a smoother patient journey, and “becoming the partner of choice for these satellite biotech organizations,” as described by the company’s Senior Director, Clinical Digital Innovation, Robert Bergann.
Bayer’s approach to clinical data transformation was guided by the philosophy: “think wild and develop resilience,” according to Bergann. This meant prioritizing what would deliver short-term impact while building toward a long-term vision of a patient-centric ecosystem.

Unlike a fixed, sequential rollout, Bayer opted for a more flexible, user-driven implementation. The company began with Veeva Study Startup and eTMF in year one, adding Veeva CTMS and Study Training in year three, before expanding to Veeva EDC and CDB two years later.
As part of the company’s migration plan to remove legacy systems, it implemented Veeva CDB with a connector to its existing EDC system, before progressively moving studies over to Veeva EDC. The standard implementation would have been to design the overall process for the full range of Veeva applications, then add the connector. This decision, driven by user impact, demonstrated pragmatism rather than adhering to a strict standard. Bergann recommends “finding an easier way to start and get started.” This approach allowed the team to gain early momentum and valuable insights.

Efficiency: Bayer redesigned its clinical data processes to enhance efficiency at scale. By strategically eliminating manual trackers and complex data conversion transformations, it anticipates significant gains, with an estimated 15-20% effort savings across operations. This focus on streamlining workflows directly contributes to faster and more reliable data handling.
Innovation: A cornerstone of Bayer’s transformation was the consolidation of its technology and processes. The team successfully replaced over 30 disparate applications and 80 integrations with a single primary platform, simplifying the technological footprint and accelerating capacity for innovation. Bayer is actively working toward a patient journey that relies on innovative trial designs and real-world data (RWD). The goal is to create a system that continues to improve the patient experience and keep them informed through the trial. This ultimately increases their chance of receiving crucial therapies.
Collaboration: Bayer’s transformation focused on its customers and end-users to ease the burden on sites and improve patients’ lives. The team recognized that trust and transparency are core components of successful clinical platform adoption. By involving these stakeholders and prioritizing their experience, Bayer ensures that the technology serves the people using it and those it aims to help.
Bayer strategically eliminated manual trackers and complex data conversion transformations, with estimated 15-20% effort savings across operations.
Starting your clinical transformation journey
Through careful consideration of the Veeva Clinical Platform and its value, Boehringer Ingelheim, Novo Nordisk, and Bayer embarked on clinical transformation journeys that align with each company’s business case:
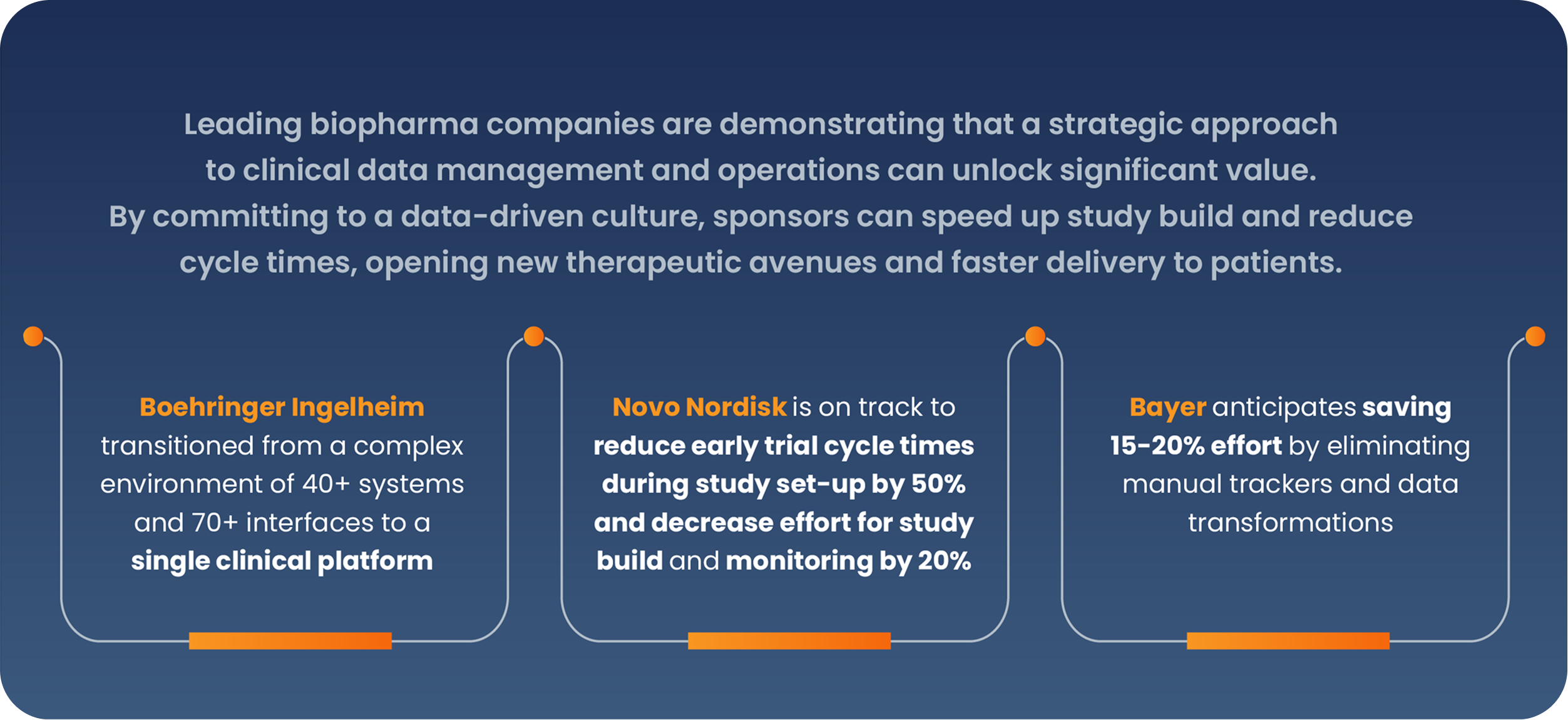
- Boehringer Ingelheim transitioned from a complex environment of 40+ systems and 70+ interfaces to a single clinical platform for clinical data, clinical operations, regulatory, and quality. The company will continue to add applications to each area and implement Veeva Safety in the future.
- Novo Nordisk is on track to reduce early trial cycle times for study set-up by 50% and decrease the effort for study build and data monitoring by 20%, with Veeva EDC and CDB.
- Bayer added Veeva EDC and CDB to its core clinical operations applications, and anticipates saving 15-20% effort by eliminating manual trackers and data transformations, streamlining processes to enhance efficiency at a global scale. The company plans to add Veeva eCOA, Payments, Disclosure, and potentially RTSM in the next two years.
A mindset shift is underway. Connected clinical systems simplify the experience for sponsors, sites, and patients, streamlining data and document flow between them.
For sponsors of all sizes considering a clinical transformation, several key takeaways emerge:

Many companies are moving towards a fully connected approach for R&D, streamlining cross-functional development processes by integrating clinical data and operations with safety, regulatory, and quality. By partnering with vendors of connected systems, organizations can accelerate development, enhance data integrity, and improve the clinical trial experience for site and patients.
Ready to transform your clinical approach? Find out how to adopt a clinical platform.
