Blog
Connecting Clinical and Field Medical to Improve the Trial Experience
Oct 05, 2023 | Dani Pagnan and Rylan Collins
Oct 05, 2023 | Dani Pagnan and Rylan Collins
90% of clinical trials fail due to a lack of clinical efficacy. But 10% of these failures are avoidable. Businesses can’t afford to throw away R&D budget on poor commercial forecasting and lack of strategic alignment. How can teams better avoid these failures?
Impact of clinical and medical teams not collaborating effectively:
- Slow trial enrollment
- Longer development timelines
- Suboptimal customer experience
- MSLs are not prepared for effective engagement with KOLs who are also PIs
*Based on survey of session attendees, Veeva Commercial Summit, North America, May 2023
Much of this can be attributed to medical teams not engaging early enough in the clinical trial protocol design, or at all. Another reason is the inability for clinical and medical teams to put together a joint strategic plan in place for site activation and management. But strengthening the collaboration between clinical and medical teams during the drug development process can significantly improve the overall process.
Medical teams excel in HCP relationship management, which can lead to better site selection, faster site activation, improved visibility during enrollment, and even early opinions on HCP adoption. This means faster, less costly, and more successful trials.
Medical teams also benefit from better collaboration and improved visibility into the company’s interactions with a principal investigator (PI).
At a recent Veeva Commercial Summit, North America workshop medical affairs and clinical operations leaders discussed the opportunities and challenges they see when looking to share information and work more closely together.
Benefits of clinical and medical collaboration:
- Clearly defined goals
- Better trial design
- Accelerated trials
- Improved patient enrollment
- Improved perception by trial site
- Improved customer experience
- Higher chance of commercialization success
- Transparency around the largest investment by biopharma
*Based on survey of session attendees, Veeva Commercial Summit, North America, May 2023
Breaking down silos
Connecting data across clinical and field medical can optimize study enrollment and accelerate trials while improving communication and coordination.
However, clinical research associates (CRAs) and medical science liaisons (MSLs) often log their activities in siloed systems, limiting visibility into key opinion leader (KOL) and HCP interactions.
A seamless flow of information between study teams and MSLs can drive collaboration and visibility to get treatments to patients faster. MSLs can share critical insights on local clinical trends and patient needs, enabling data-driven decision-making by clinical study teams. Data shared by CRAs, such as calls or on-site visits, give MSLs a 360-degree view of interactions, which helps strengthen their relationships with HCPs and identify future endorsement opportunities. This also leads to a more consistent and superior customer experience for principal investigators who are also KOLs.
Why the disconnect?
Workshop participants identified cross-functional data sharing as the number one issue that’s getting in the way of working more collaboratively. This was followed by a lack of clearly defined benefits and visibility into key clinical trial milestones.
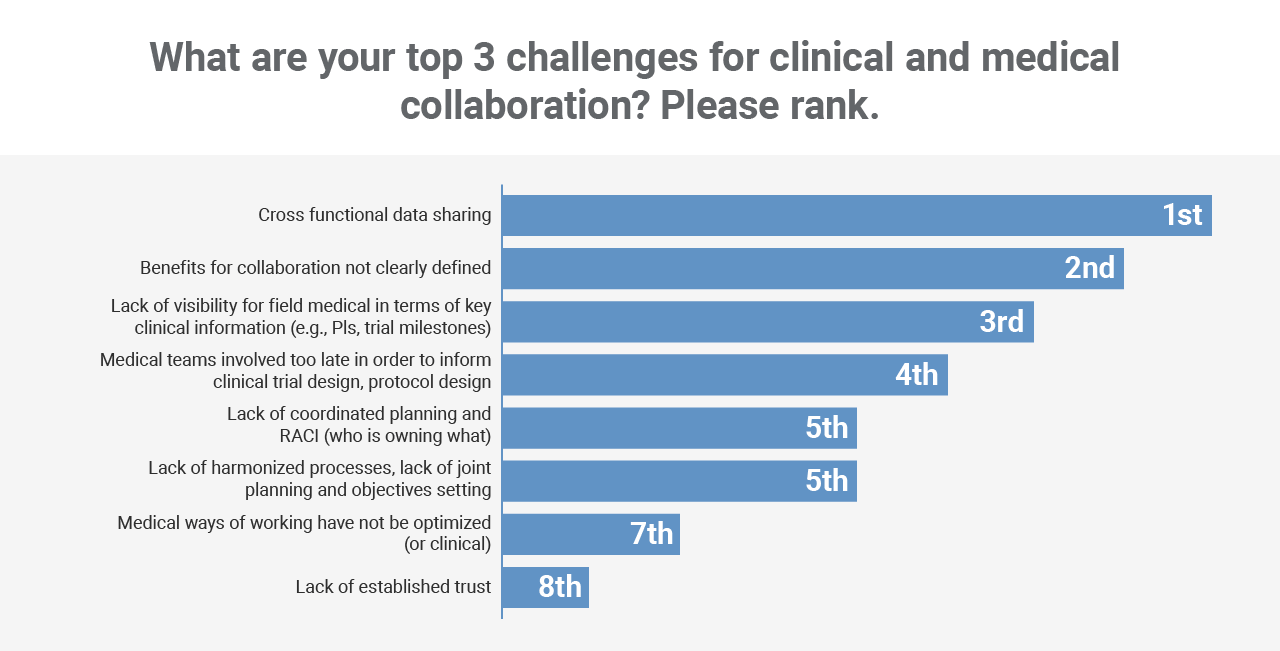
*Based on survey of session attendees, Veeva Commercial Summit, North America, May 2023
Building a new coordinated approach for clinical and medical teams is a journey that requires a detailed plan with defined immediate actions and a long-term roadmap. It encompasses technology, data, and process improvements.
Technology
As activities with HCPs are planned and completed, both MSLs and CRAs need full visibility and a complete history of interactions. Connecting Vault CTMS with CRM enables the seamless flow of information between clinical and medical teams.
Data
A common ID can connect customer information between clinical operations and CRM solutions, even when customer master data may differ. This allows for complete transparency and alignment on activities logged against a study, site, or person.
Process
Improving collaboration between clinical and medical teams is an operational reality but doing so in a scalable and sustainable way requires clear change management. Make sure to define the benefits as well as the process. What are the specific trial milestones, and when should field medical teams get involved? What additional insights could be provided to benefit both clinical and field medical teams? There must be a coordinated plan and ownership with joint objectives.
Workshop participants noted that regular, unified meetings and working sessions between clinical and medical teams would be needed to best collaborate.
Where to begin
There is a significant runway for increasing collaboration between clinical and medical teams. So, how should biopharma organizations get started?
- Articulate the “why” – Define a shared value proposition and goal across clinical and medical teams to increase collaboration and improve the site and patient experience.
- Start small and then scale – Begin with the minimum viable model of business process change needed on both sides and keep the investigator/KOL at the center. Enable process change successfully, measure and quantify the difference, then scale.
- Explore technology – Assess how technology can help strengthen collaboration between clinical and field medical teams and streamline data capture and processes.
To learn more about connecting clinical and medical affairs to improve the trial experience connect with Veeva Business Consulting.
