Blog
Growing Risk Management Findings: How to Get Ahead and Improve Compliance
Feb 28, 2025 | Christina Kim and Celine Ghafari
Feb 28, 2025 | Christina Kim and Celine Ghafari
Welcome to Veeva’s Safety Newsletter. In this edition, learn why risk management is a top source of safety inspection findings and how to enhance your approach to global risk management.
Risk management is a top source of inspection findings
Consistently resulting in the largest number of MHRA overall findings reported since 2012, risk management continues to be a top challenge.
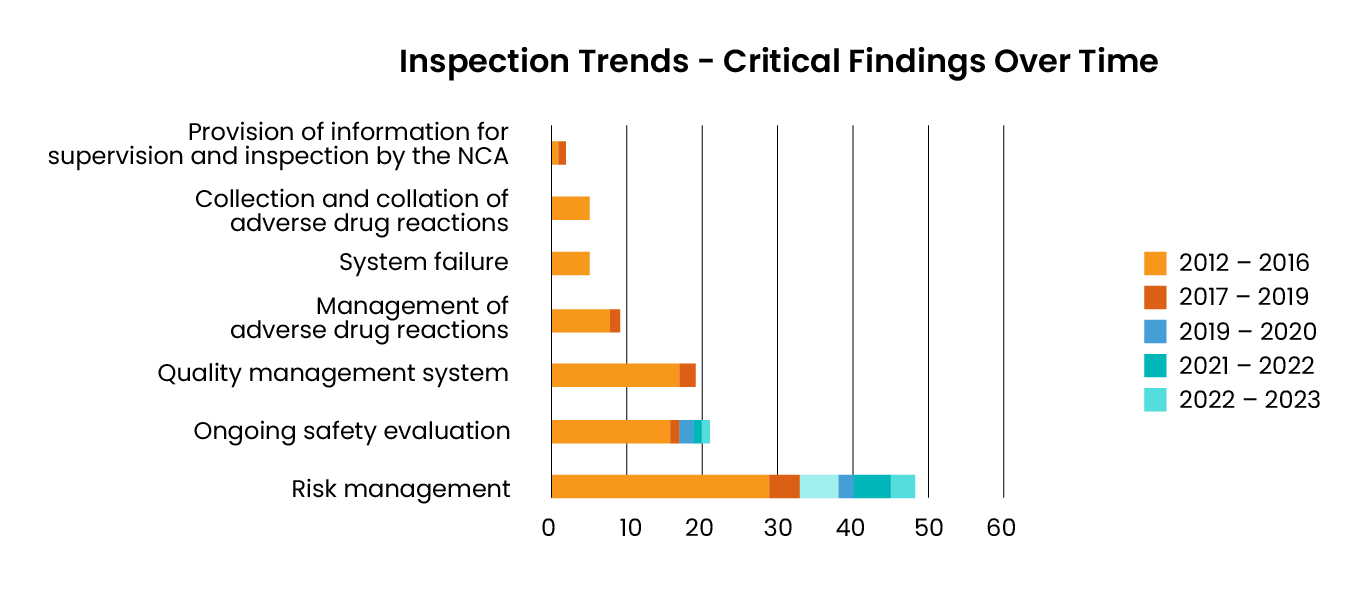
With fragmented processes, spreadsheets, and manual trackers, it is difficult to manage risk and stay compliant.
Biopharmas are investing in improving global risk management
Innovators are enhancing risk management approaches with modern safety solutions that can:
- Unify safety information and automatically share with relevant parties internally and externally (CROs, affiliates, service providers) such as reusing core RMPs in local versions
- Easily manage and have oversight of an RMP for a product and market
- Facilitate timely implementation of aRMMs, track efficacy, and monitor commitments and compliance
“Safety data impacts key decisions, like whether or not a trial will continue, or whether there’s a need for increased risk mitigation strategies,” says Hanssar Chacon, senior director of global PV and risk management at CRISPR Therapeutics. “It’s critical to be able to look at the data, understand it, and pick up signals before regulators do to find the best way to minimize potential risks.”
Streamline Risk Management and Risk Minimization Measures
See how to improve oversight and efficiency in global risk management.
Watch the videoTo receive a monthly Safety newsletter in your inbox, subscribe here
To read more Safety newsletters, click here.
