Blog
Reduce Case Rework and Increase Data Quality by Improving Case Processing Workflow
Mar 31, 2025 | Christina Kim and Celine Ghafari
Mar 31, 2025 | Christina Kim and Celine Ghafari
This edition of Veeva’s Safety Newsletter highlights the importance of quality control (QC) checklists in an end-to-end case processing workflow.
Embedded QC to increase data quality in case processing
Although speed is essential in case processing, inadequate QC can compromise case quality. Incomplete or inaccurate QC checks raises the risk of case rework and failed ICSR submissions to regulatory authorities, increasing the workload for medical reviewers, and extending case processing time by 25% or more.
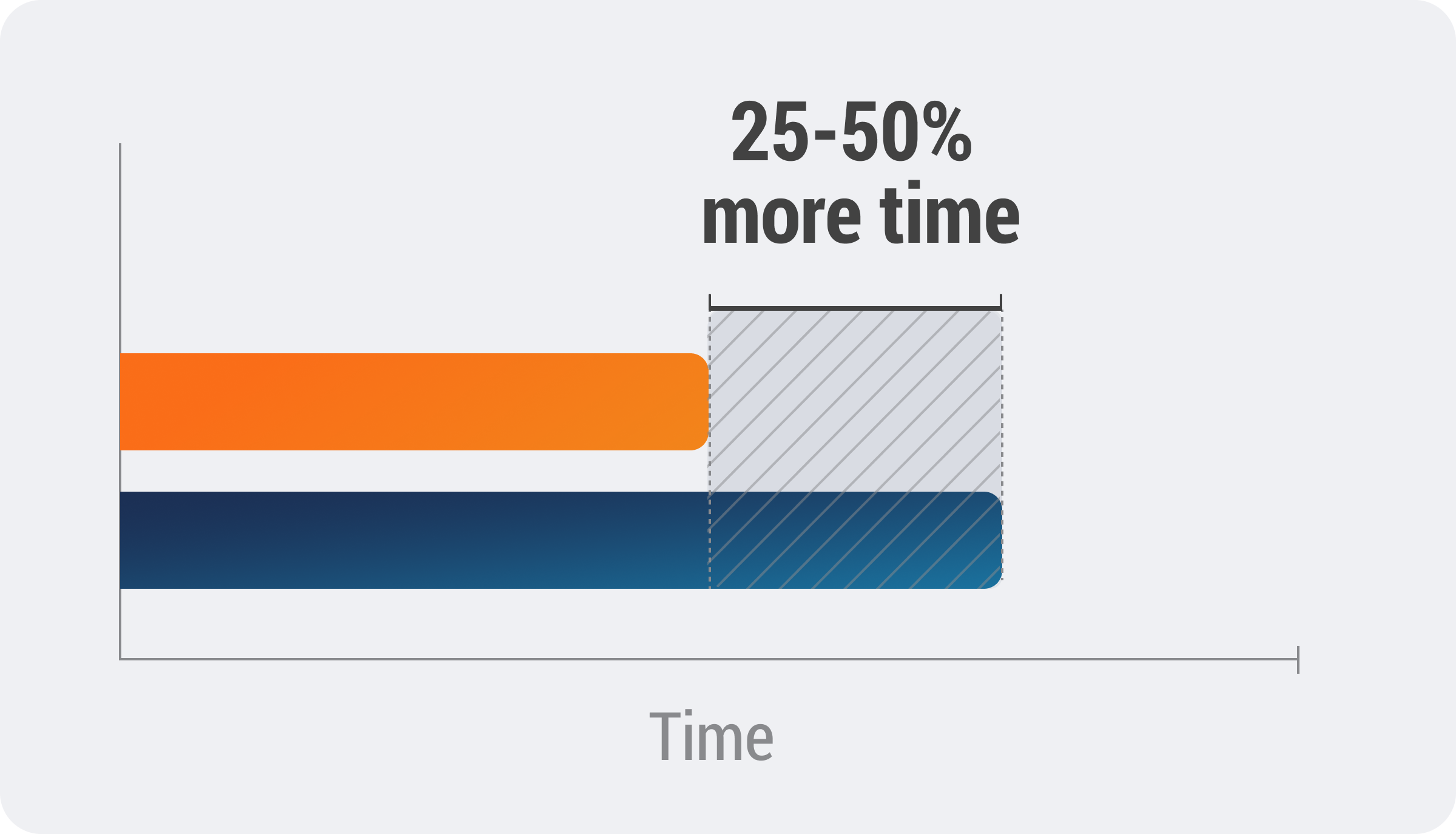
To reduce case rework and improve data quality, many biopharmas use a QC process that relies on individual spreadsheets to capture QC data. These spreadsheets make it difficult to consolidate QC results and track trends to address sources of frequent data issues.
Driving quality excellence with embedded QC workflows
Embedding quality control (QC) checklists into case processing helps identify sources of quality issues, track QC result trends, and measure the effectiveness of supplemental training.
Streamline Case Processing with QC Checklists
Demo on how Veeva Safety QC checklists are automatically incorporated into case processing workflows to improve efficiency and data quality.
Watch the video“We had to redo a lot of cases after they came to us for review. We would train people, but our studies are complex and the medical review is very challenging,” said the global head of PV and risk management at a global biotech. See how an embedded QC checklist improves data quality.
Achieving seamless end-to-end case processing
Pharmacovigilance (PV) teams benefit from end-to-end case processing with a modern safety solution that:
- Incorporates low-touch case processing and supports multilingual local ICSR reporting in one global PV solution including seamless submissions and tracking to PDMA, FDA, MHRA, and EMA.
- Provides real-time safety reports and dashboards for tracking case processing time, case quality, and regulatory reporting compliance.
- Automates the flow of serious adverse event (SAE) events from clinical data to safety systems for streamlined case processing and reduced data reconciliation.

Streamlining Processes with Unified and Connected Safety
“The opportunities we (Merck) have to make our processes more efficient and reduce burden in terms of multiple data entry in quality checks is enormous.” – Cory Gilbert, Senior Director, PV Operations and Global Process Enablement, Merck
Watch the videoTo read more, visit Safety newsletters.
