Blog
Supporting EMA’s New IDMP Implementation Guide 2.0
Apr 14, 2021 | Kelly Finlan-Dansbury
Apr 14, 2021 | Kelly Finlan-Dansbury
After several years of delays and ongoing uncertainty about ultimate compliance deadlines, the EMA recently released a new Implementation Guide (EU IG v2.0) for its Identification of Medicinal Products (IDMP) standard.
In response to these new guidelines, our Vault RIM development team is currently building out functionality to support compliance while best serving our customers’ ongoing needs. At Veeva, we expand our products based on published guidance whenever possible, rather than working speculatively. This reduces the burden of rework on our customers, as well as our developers. Through the use of an agile development methodology, our product team continually delivers new code packages and adjusts scope based on circumstances changing outside of our control.
Based on the previous guidance from EMA (IG v1), we already have several pieces of the IDMP framework in place including a data model, process automation, data view UI design, data record automation, and integration with the SPOR RMS database.
Next, our Vault RIM team will prioritize submission readiness, which involves building a connection to the PMS database and functionality to generate the submission XML (FHIR Package).
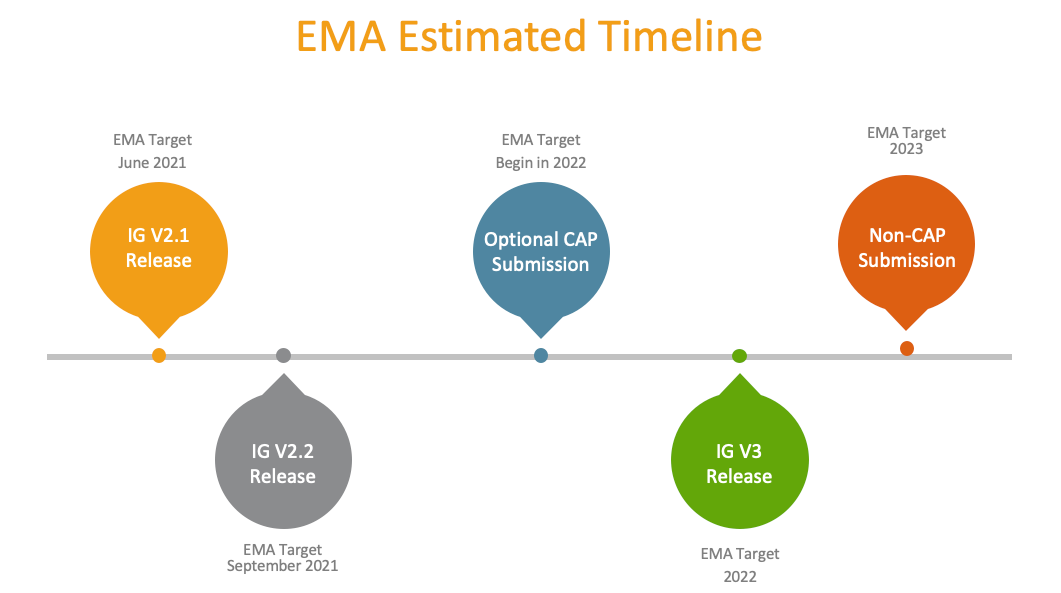
EMA’s Estimated Timeline of Events and Veeva Support of XEVMPD
While EMA’s timeline may well slip in the future (as it has in the past), here are the key milestones for biopharma organizations to consider:
During this transition period, EMA will continue to accept XEVMPD submissions and we will continue to support them in Vault RIM through capabilities including acknowledgment, EV code, and validation results handling.
Fortunately, customers will not have to worry about migrating XEVMPD data to the PMS database, as this will be handled by EMA as part of the transition process.
What Industry Can Do Now to Prepare
While you cannot submit to the PMS database until EMA is ready to accept your data and Vault RIM is ready to support it, there are important steps that Vault RIM customers can take now to prepare for the challenge ahead.
Every organization affected by IDMP should be performing data readiness assessments and establishing internal data governance standards for regulatory and supply chain data. Ensure you know the scope of the data collection process, who owns what datasets, and how the process will be managed when the time comes. You may find a great deal of uncertainty exists within your organization between departments that will need to be ironed out in advance of the data collection effort.
You will likely also find that data remediation projects are required in order to clean your data, reconcile disparate data sources, and augment missing fields.
These are all steps you can take now to prepare your data and your team to meet the challenge of IDMP in 2022 and beyond.
Visit our Resource Centre to get the latest insights on innovative approaches to digitization from leading pharma and biotech companies.