Veeva MedComms
Content Management Tailored for Medical Affairs
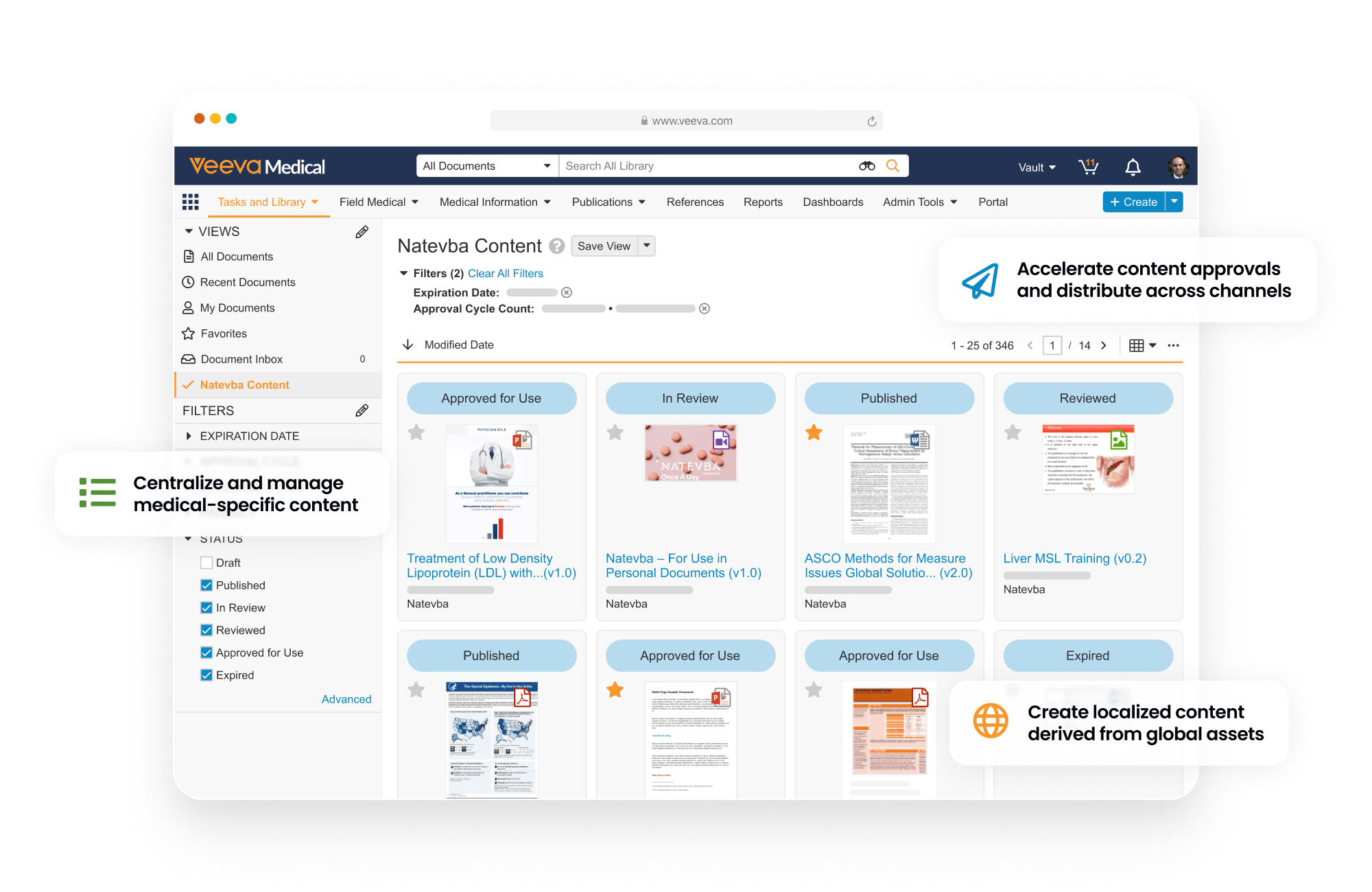
MedComms is a regulated content management application that supports unique content types, lifecycles, and workflows specific to the needs of Medical Affairs. It enables content creation, review and approval, storage, management, and distribution.
Connecting to CRM for automates distribution of medical content via applications like CLM and Approved Email. The MedComms API enables integration with third-party systems.
Core capabilities include Medical Portal—a central location to showcase curated content, enhancing accessibility and content reuse—and Scientific Communication Platforms which define scientific position, narrative, and objectives, as well as scientific statement and reference libraries.
Announced 2012 Status Very Mature Customers 100+
Measuring Medical Impact - Best Practices and Metrics for Targeted Scientific Education
Overview
Content Management Tailored for Medical Affairs
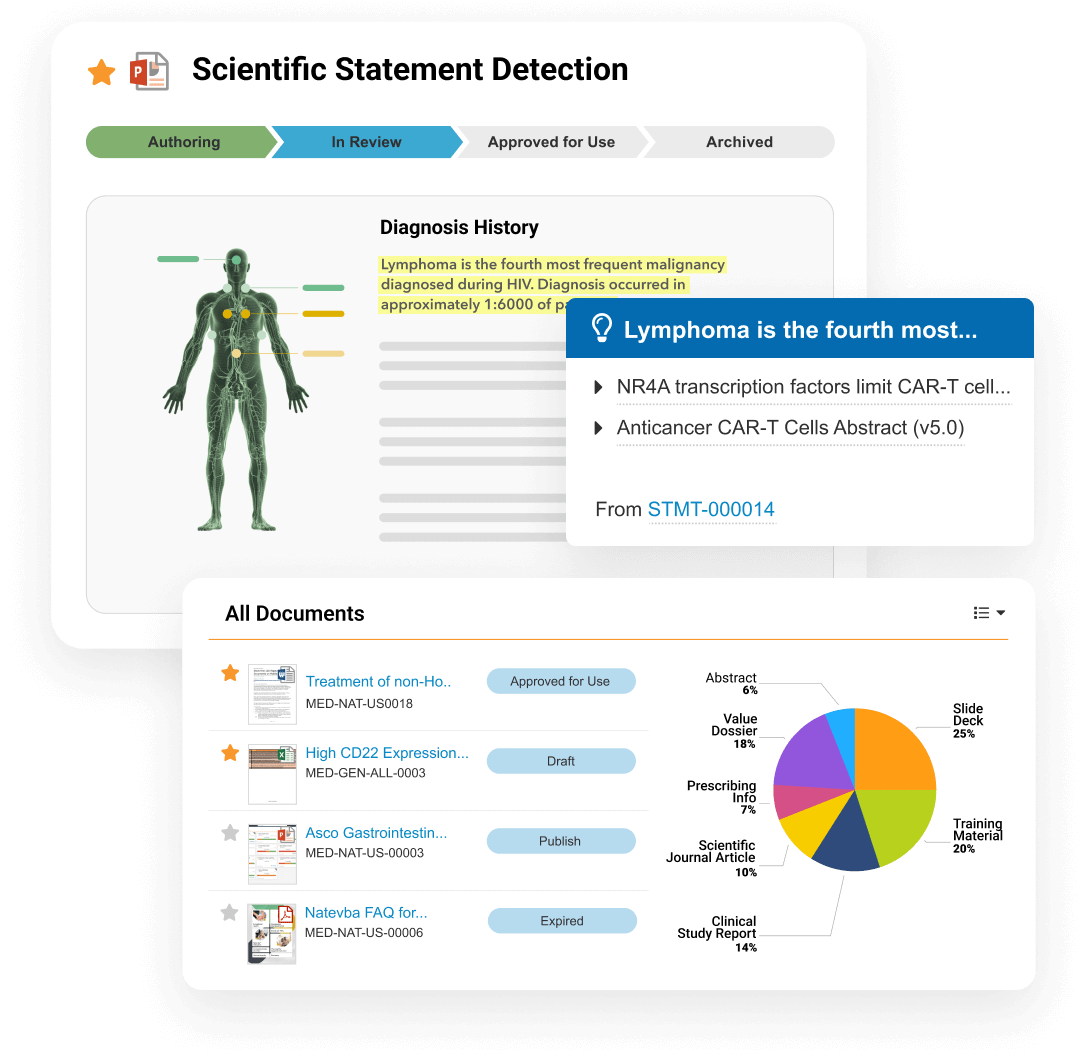
MedComms is a regulated content management application that supports unique content types, lifecycles, and workflows specific to the needs of Medical Affairs. It enables content creation, review and approval, storage, management, and distribution.
Connecting to CRM for automates distribution of medical content via applications like CLM and Approved Email. The MedComms API enables integration with third-party systems.
Core capabilities include Medical Portal—a central location to showcase curated content, enhancing accessibility and content reuse—and Scientific Communication Platforms which define scientific position, narrative, and objectives, as well as scientific statement and reference libraries.
Why Veeva MedComms
Centralize and scale medical content
Customer Success
The industry standard for
medical content management


















Resources
Explore and Learn
Read Features Brief
Veeva MedComms Features Brief

Read eBook
Digitizing Your SCP: a Modular Approach to Medical Content

Watch Video
Achieving Medical Content Excellence with Veeva MedComms

Watch Video
Idorsia Builds a Medical Content Strategy for High Growth

Watch Video
Merck’s Global to Local Approach to Medical Content

Read eBook
Transforming Medical Content for Omnichannel Success

Read White Paper
Measuring Medical Affairs’ Impact: Targeted Education

Watch Demo
Streamlining Medical Content for Effective Scientific Communications

See Innovation Guide
Making the Most of Veeva MedComms

Watch Video
Enabling MSLs with the Right Content at the Right Time

