Veeva QMS
Streamline and Simplify Quality Processes
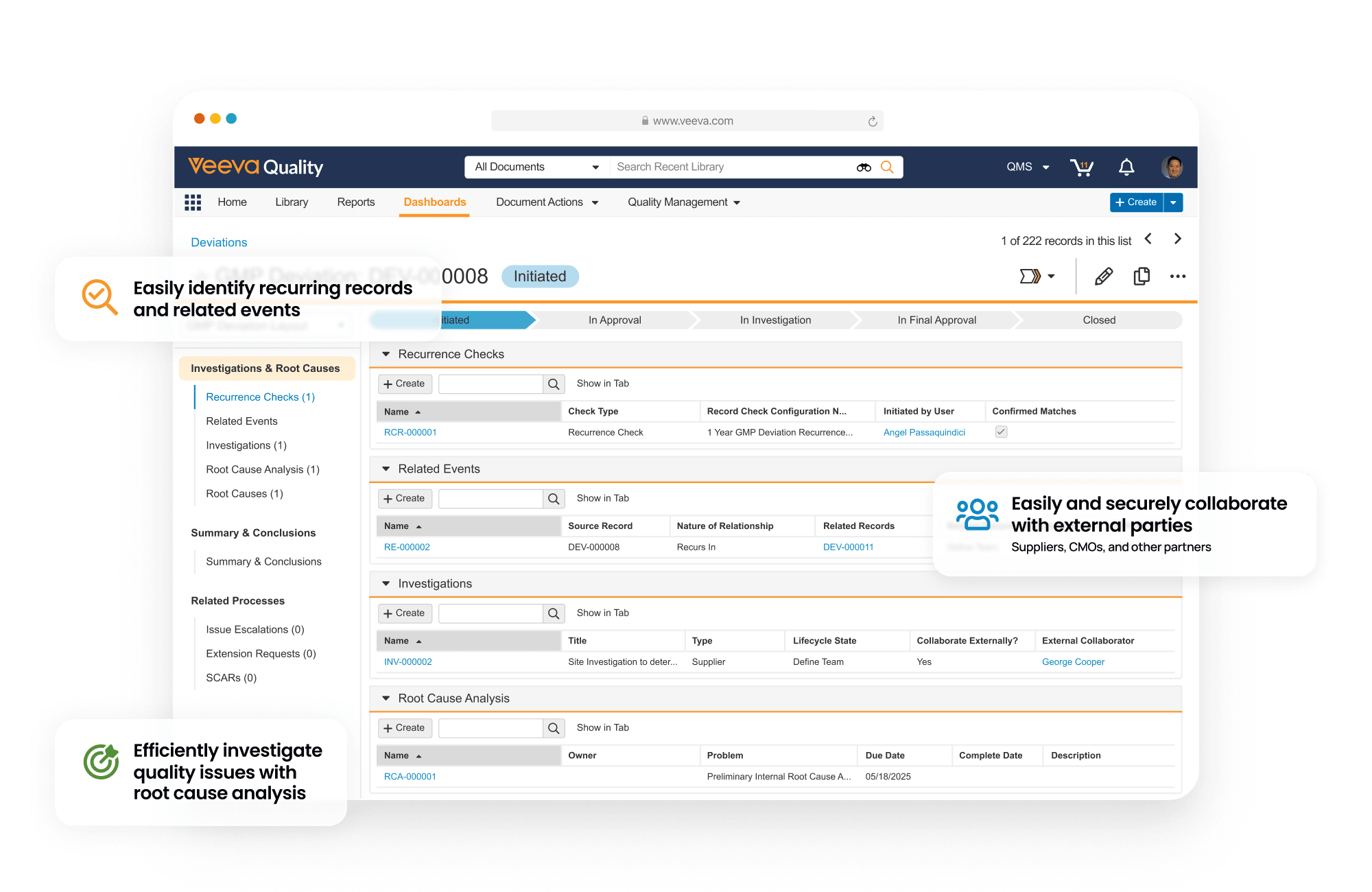
QMS is a cloud based quality management system designed to manage life sciences-specific quality processes. It provides faster time to value with streamlined processes for handling complaints, deviations, audits, quality risk management, supplier quality management, and change control. It also allows external partners to access the system in real time to collaborate on investigations, audit findings, corrective actions, supplier change control and more.
QMS is unified with other Quality Cloud applications, and connects to RIM to coordinate product change control activities, Safety to manage complaints, and CTMS for study-related data used in QMS and protocol deviation management.
Announced 2016 Status Very Mature Customers 100+
Simplifying quality management with Veeva QMS
Overview
Streamline and Simplify Quality Processes
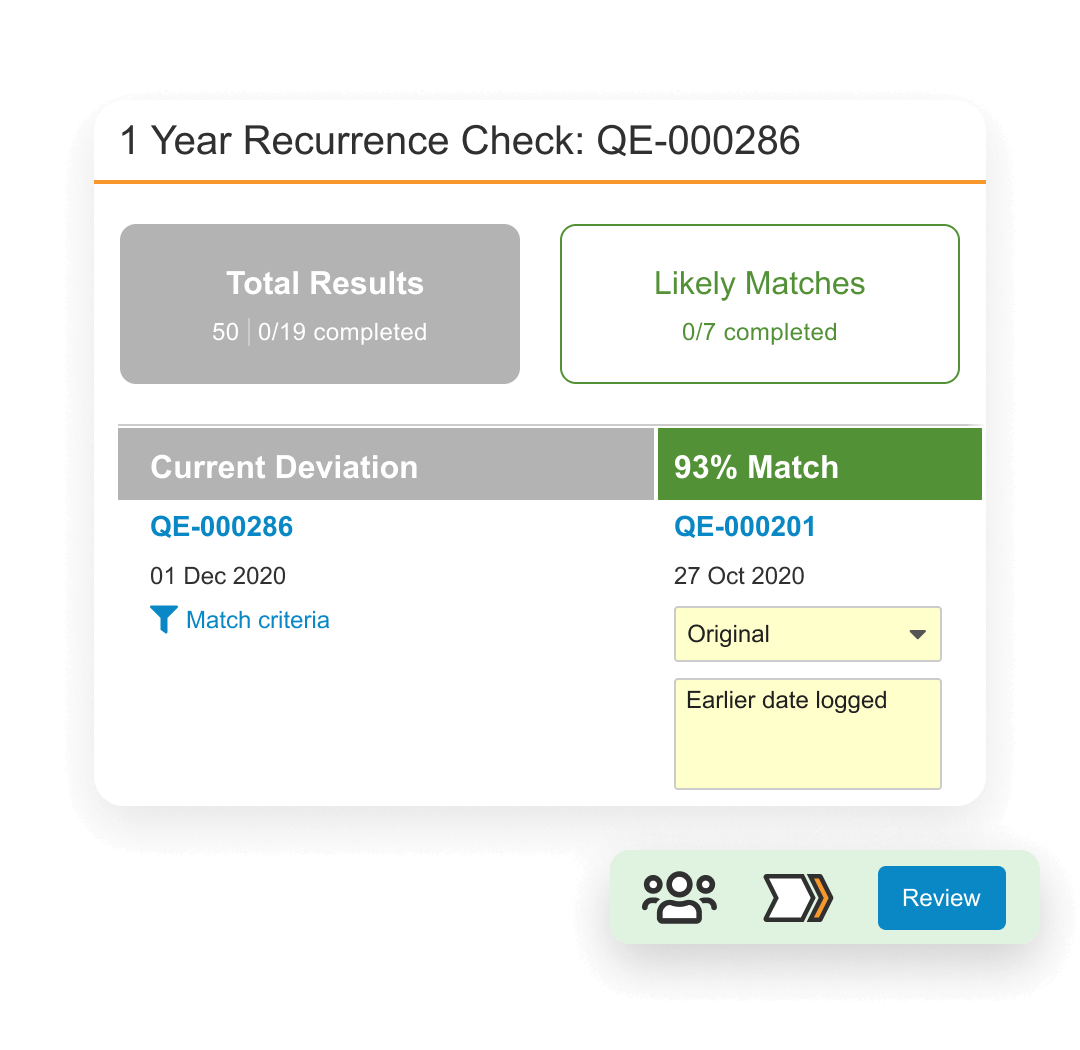
QMS is a cloud based quality management system designed to manage life sciences-specific quality processes. It provides faster time to value with streamlined processes for handling complaints, deviations, audits, quality risk management, supplier quality management, and change control. It also allows external partners to access the system in real time to collaborate on investigations, audit findings, corrective actions, supplier change control and more.
QMS is unified with other Quality Cloud applications, and connects to RIM to coordinate product change control activities, Safety to manage complaints, and CTMS for study-related data used in QMS and protocol deviation management.
Veeva AI for Quality
See in Action
Quality Event Agents
Aggregate data across multiple objects to generate narrative summaries for Investigations and CAPA plans.
Document Translation Agent
Generate translations of documents such as SOPs into various languages to improve overall cycle times.
Why Veeva QMS
Faster time-to-value with streamlined processes
Customer Success
More than 300 companies streamline
quality processes with Veeva QMS










