eBook
How Emerging Biopharma Can Launch
and Scale Content Faster
Four best practices to creating a commercial content foundation from medical,
legal and regulatory (MLR) review to delivery across channels
The rapid creation, review, and approval of promotional assets can be a key competitive advantage
for pre-commercial companies. The quality of the foundation you lay for your content workflow now,
including the medical, legal, and regulatory (MLR) review process, will help your company to secure
that competitive edge and scale more efficiently in the future.
One of the unexpected benefits of healthcare professional (HCP) engagement going digital was the opportunity for reps to spend more quality time with doctors.
Virtual meetings extended the amount of time
HCPs were able to meet with reps from an average
of three minutes to 19 minutes, when compared
to traditional, face-to-face meetings.

Rep-sent email also drove significant engagement,
with an average open rate of 36%.
While the chance to engage in more meaningful conversation has certainly been a positive thing for field teams, it’s also created a greater need
for marketers to produce more content, faster. Establishing the right content model early on is key for emerging biopharma companies looking to
enable and sustain rapid content creation at scale.
The first step is to clearly outline how your organization will manage its assets through each stage of the content lifecycle. From creation,
review, and approval, to distribution and delivery, think about how your content will ultimately make its way to the hands of doctors and patients
in the most efficient—and compliant—way possible. The following best practices can help get the ball rolling and ensure that you’re building a
long-term content foundation that can scale with your business.
"We wanted a system that was suitable for every country but didn’t have to be overly
customized. Vault PromoMats gives us the global collaboration and automated audit
trails we need, while gaining the flexibility to adapt to edge cases as they arise."
Agnès Keltie, Norgine
1. Establish a single source of truth for all content stakeholders

Before the content creation process begins, your commercial team and agency
partners will want to establish a comprehensive launch strategy as far in advance
as possible. This strategy should include a content plan with key assets to be
created, as well as early concepts that they can share with the medical, legal,
and regulatory (MLR) review team before content development begins. Ensuring
all stakeholders are working and commenting on the same content will reduce
inefficiencies and accelerate content to market.
Make sure that anticipated launch dates are continuously communicated so the
marketing team has enough time to include an initial round of concept review
ahead of content creation. Not only will this help reduce the number of iterations
and review cycles the team has to go through later on, but it will ensure final
assets are approved and ready to go as soon as your product has been cleared
by regulatory authorities.
2. Fast-track MLR reviews with automation

Once content plans are approved and assets are drafted, the next phase of the
content lifecycle is review and approval. Marketing will need to route all assets
to necessary stakeholders to ensure that they are compliant and ready for
distribution—a process that can quickly become complicated.
Email and spreadsheets are often a starting point for emerging companies.
However, they need to be managed and manually updated constantly, making it
easy to lose track of where assets are in the review process. The result is a loss
of precious time and money, as well as an increase in compliance risk.
A simpler and more sustainable option is to automate these cycles through a
cloud-based MLR review system. With review and approval being tracked in one
place, stakeholders will have an easier time managing approval queues while also
gaining visibility into what’s coming down the pipeline. Meanwhile, marketing
and commercial operations teams will be quickly able to spot any bottlenecks in
the process and ensure that assets are not only approved but submitted to the
appropriate regulatory bodies in a timely manner.
"To deliver great customer experiences with content, we should be able to provide
what the customer wants when they want it, and where they want it. We have set out
to replace the traditional ‘push to consumer’ content model with a customer-centric
engagement one."
Mubasher Hassan, Grünenthal
3. Optimize channel mix for maximum impact

After content has been approved, it’s now time for the commercial team to
educate the market with brand-compliant messaging and materials. The volume
of your content will only grow as it gets pushed through more channels across
multiple audiences, so make sure the team has an easy way to manage channel
content distribution and delivery.
In addition to in-person meetings, consider a variety of digital channels such as
your website, social media, newsletters, webinars, and even an online event to
distribute content. Most cloud-based content management solutions will let you
create, approve, and distribute content all from the same platform, while also
helping with content withdrawal when needed.
Label changes or new drug studies will require rapid updates to all promotional
and medical content, typically within a three- to six-month timeframe. To avoid
being penalized, make sure you have visibility into all content and all channels that
the assets are being distributed from. Leverage your content system to ensure
that only approved materials make it out to market and maintain control of assets
with instant withdrawal, controlled updates, and automatic expiration.
4. Measure performance and adjust content strategy as needed
Getting your content in the hands of your customer is a great milestone, but it’s
not the final step. The marketing team will need to understand which materials
are resonating with your audience and through which channels, whether that’s
patients on your website or doctors in an office.
Measure customer reaction to and engagement with assets using tools like
tracking links, social metrics, and content sentiment, all of which should flow
back into and inform your centralized content strategy. It’s also a good idea for
reps to ask for feedback directly, especially when meeting with HCPs in person.
Once you gather data on asset performance, you can analyze your results and
use these findings to adjust your marketing tactics moving forward.
Planning for the future
The rapid creation and management of promotional and medical assets is a key competitive advantage for pre-commercial companies.
The efficiencies you build into your content workflow now will help your company scale more efficiently using new approaches like modular
content, now or in the future.
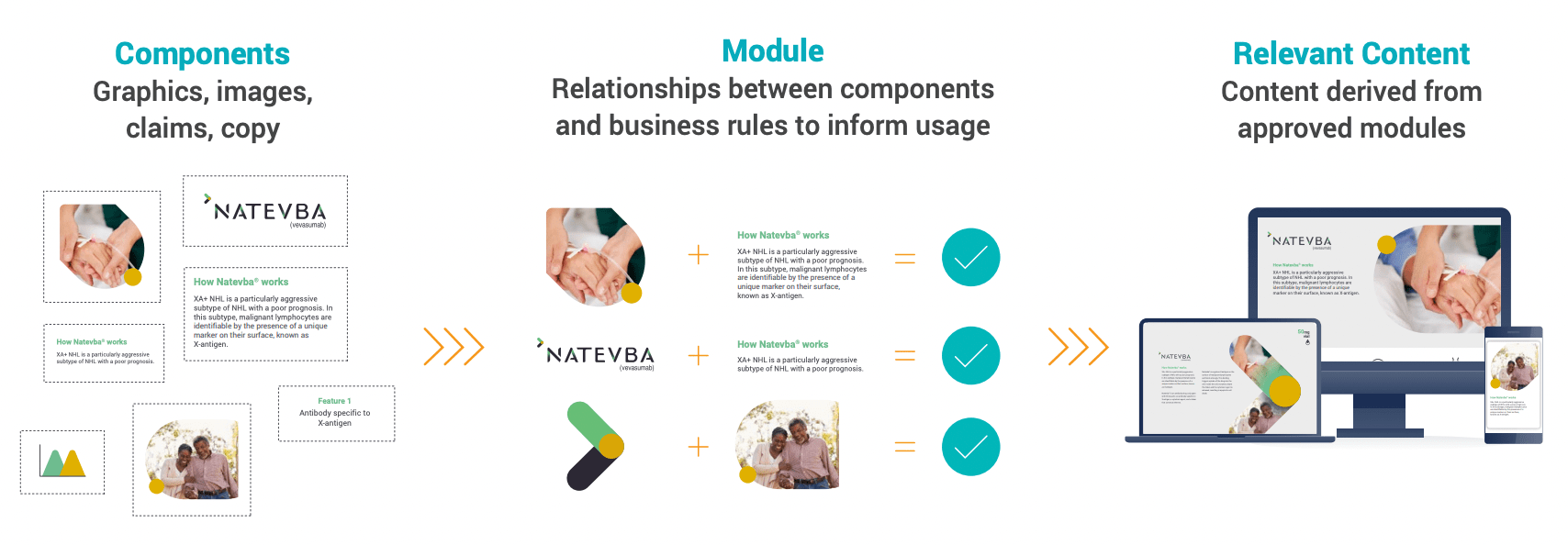
A modular content approach reassembles existing, pre-approved content blocks, or modules, into a variety of assets that can be used in
different channels and regions. It can be particularly useful for fast-tracking content creation and approval, in addition to achieving the ultimate
goal of personalized content.
MODULAR CONTENT
"My advice to companies looking for a commercial content solution is,
set it up early, and leverage all the best practices so you can hit the ground
running and have a scalable solution for the future."
Darran White, Upsher-Smith Laboratories
Learn more about launching and scaling content faster.
