Features Brief
RIM-Clinical Operations
Connection Features Brief
Although they focus on vastly different use cases, clinical and regulatory teams leverage hundreds of overlapping documents and data points. However, this information often exists in disparate systems, which makes it time-consuming to send back and forth and creates confusion as to who owns what. As a workaround, biopharma companies create custom integrations to join this data together, but those are resource-intensive and costly to maintain.
In response, Veeva developed an integration between clinical and regulatory Vaults so teams can automatically share documents and data.
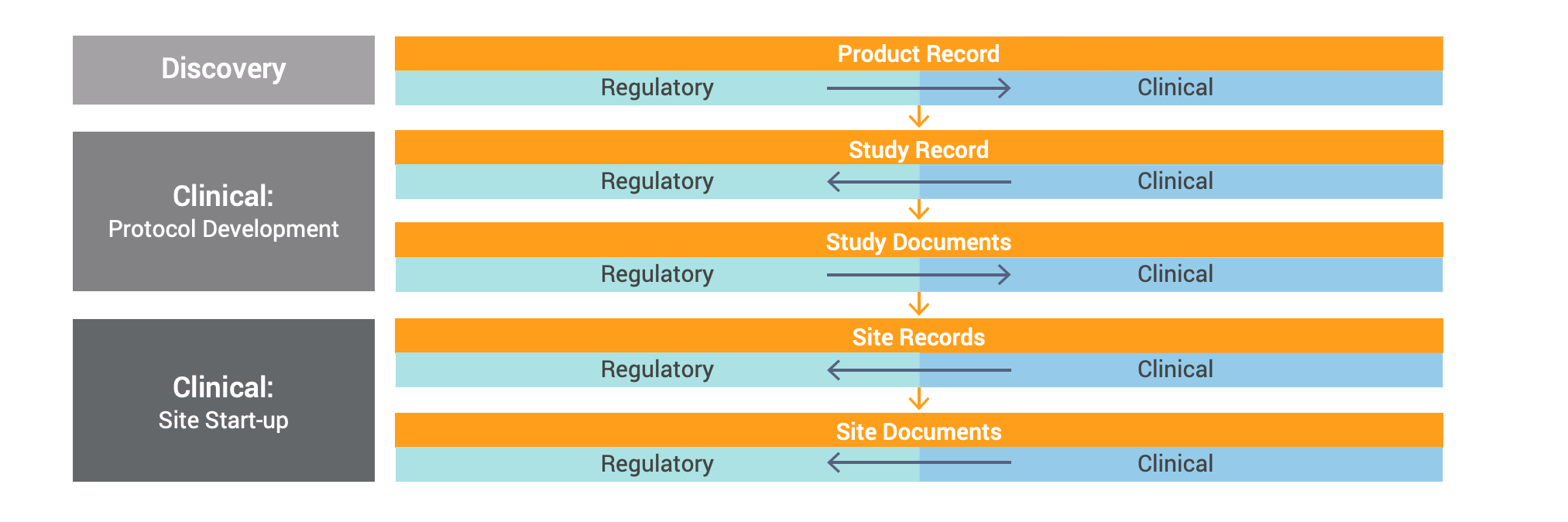
How it Works
The RIM-Clinical Operations Connection enables the seamless flow of information through automatic object data exchange and document cross-linking. The clinical Vault continues to be the master of clinical study, study product, site data, and other documents authored by that team, while the regulatory Vault is the master of product family records along with published outputs required for eTMF tracking and documents authored by that team.
Vault users are able to configure certain aspects of the connection, and it’s flexible enough to accommodate different document types and changes in direction, if needed.
Benefits
By implementing this connection, Veeva customers improve communication between Vaults and enhance end-to-end process visibility. When clinical teams compile documents for an eTMF inspection, they can be confident that they’re using the latest versions. Similarly, as regulatory teams assemble submissions for health authorities they no longer have to worry about redundant data entry or duplicate work that can produce errors and increase risk of non-compliance.
To see the RIM-Clinical Operations Connection in action, watch this demo
