Features Brief
Veeva CTMS Risk-based
Study Management Features Brief
Risk-based Study Management

With a renewed focus on risk-based approaches to clinical trial management, regulators are increasingly emphasizing critical data and processes, and encouraging extended use of centralized monitoring to improve patient safety and trial quality.
Risk-based study management (RBSM) is Veeva’s flexible approach to clinical risk management that applies to all aspects of a study. Our solution enables sponsors and contract research organizations (CROs) to assess and mitigate risks at the study, country, and site levels, with configurable workflows embedded directly within Veeva CTMS and unified with Veeva eTMF. With a seamless user experience and the ability to track and manage issues, companies can decrease site monitoring costs and improve study oversight.
Benefits
-
Improve Data Quality
Allocate valuable resources to critical data review and monitoring sites that need the most attention. -
Reduce Operational Risk
Identify timeline and execution risk, then take corrective actions to keep trials on track. -
Increase Efficiency
Assess, evaluate, mitigate, and remediate risks within Veeva CTMS for true closed-loop issue management capabilities.
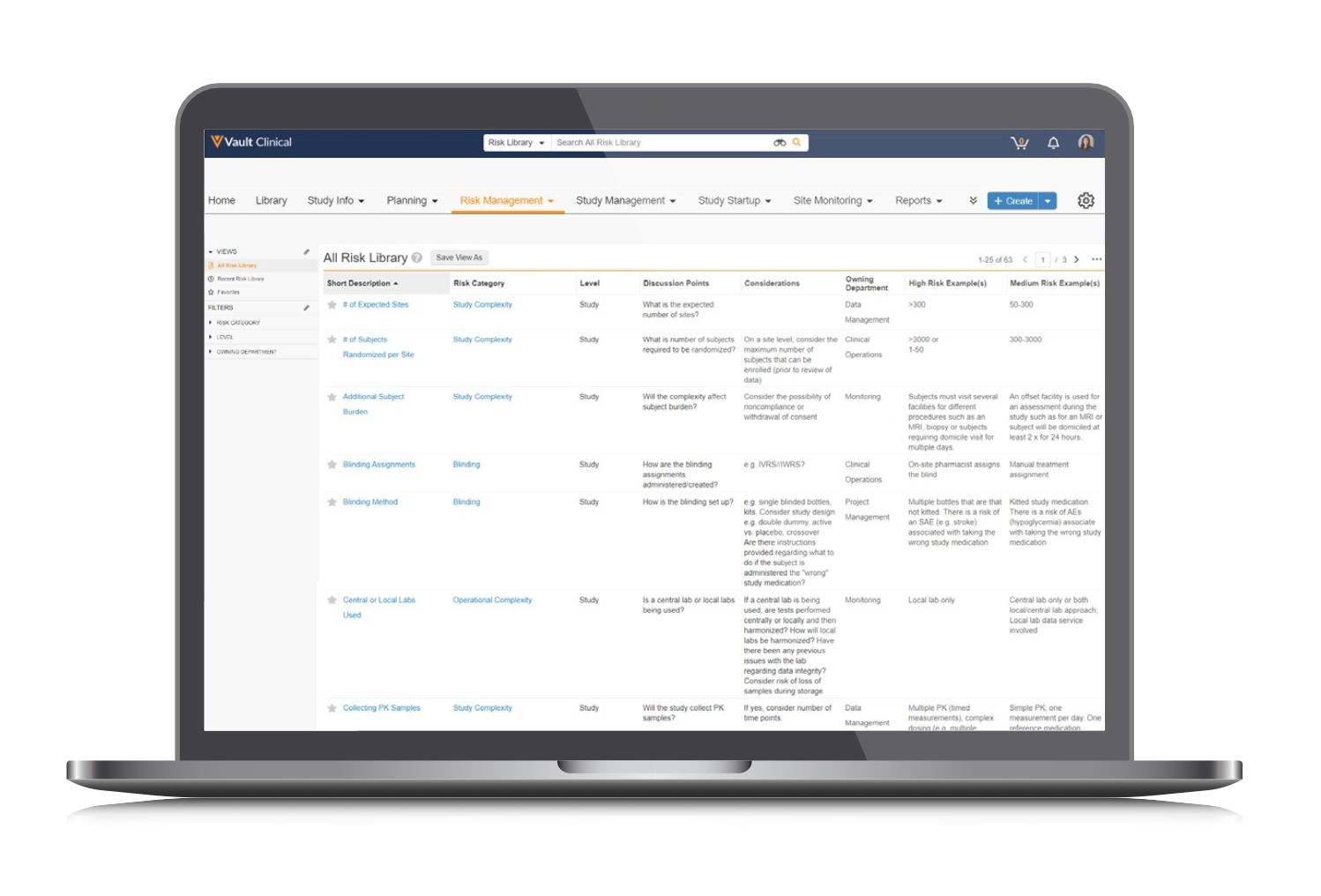
Risk Library
Centralize risks and perform holistic cross-functional reviews by creating and managing risks in the risk library. Import risks to your library as a starting point, then reuse them across studies using risk templates.
Critical Data and Processes
Define data points and processes that are critical to study execution, apply to study risks, assess the impact on downstream activities, and monitor throughout the trial.
Risk Assessment Templates
Collaborate with study teams, mitigation owners, data management, stats, and other functions to create risk assessment templates for specific phases or therapeutic areas that can be used across studies.
Study Risk Assessments
Add risks from the library to study risk assessments to create study-specific risks that can be modified, scored, reviewed, and approved. Veeva CTMS calculates the risk score based on impact, probability, and detectability, and automatically generates a risk assessment document that is classified and filed in Veeva eTMF. All changes are tracked through periodic review for a complete audit trail.
Mitigations
Manage all of the actions taken to prevent a risk from occurring in the risk mitigation library. Relate them to risks in your risk library or create study-specific mitigations. Determine if mitigation types require action item follow- ups or tracking only, and close the loop by assessing if items are resolved.
Reports and Dashboards
Organize, analyze, and share data with interactive reports and dashboards. Get visibility that drives action by tracking the riskiest sites and studies, identifying the most problematic risks across studies, and more.
